Exam 13: Structure and Shape
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
Which of the following has a tetrahedral electron pair geometry surrounding the central atom?
(i)PH3
(ii)BH3
(iii)BeH2
(iv)OF2
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
E
Which of the following is the best Lewis diagram for HCO3-?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
E
Which of the following molecules is/are polar?
(i)HF
(ii)BH3
(iii)CBr2F2
(iv)PF3
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
D
Consider the following general wedge-and-dash diagram: 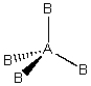 What is the molecular geometry around the central atom of this molecule?
What is the molecular geometry around the central atom of this molecule?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following wedge-and-dash diagrams best illustrates the molecular geometry of C2H6?
(Multiple Choice)
4.9/5  (37)
(37)
What is the electron pair geometry surrounding the boron atom in BH3?
(Multiple Choice)
4.9/5  (36)
(36)
What is the molecular geometry surrounding the carbon atom in CH4?
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following statements is/are correct?
(i)A molecule is nonpolar if it contains only nonpolar bonds
(ii)A polar molecule is one in which there is an asymmetrical distribution of charge.
(iii)If the central atom in a molecule has no lone pairs and all the atoms bonded to it are identical,the molecule is nonpolar.
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following types of compounds best describes organic compounds?
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following molecular geometries yields molecules which must be nonpolar?
(Multiple Choice)
4.9/5  (39)
(39)
Consider the following general wedge-and-dash diagram:  Which of the following has this structure?
Which of the following has this structure?
(Multiple Choice)
4.9/5  (40)
(40)
Consider the following general wedge-and-dash diagrams. (i)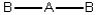 (ii)
(ii)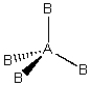 (iii)
(iii)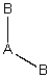 Each consists of a central atom bonded to 2 or 4 terminal atoms that are identical to one another. The central atom is different from the terminal atoms. Which of these molecules is/are polar?
Each consists of a central atom bonded to 2 or 4 terminal atoms that are identical to one another. The central atom is different from the terminal atoms. Which of these molecules is/are polar?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is the best Lewis diagram for HCO2-?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following molecular models has the same molecular geometry as that surrounding the oxygen atom in OF2?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following has a(n)bent (angular)molecular geometry?
(Multiple Choice)
5.0/5  (39)
(39)
What is the electron pair geometry shown in the following model? 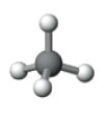
(Multiple Choice)
4.8/5  (37)
(37)
A molecule consists of a second period central atom with three other atoms singly bonded to it.There is one lone pair of electrons on the central atom,and the other atoms have lone pairs.What is the molecular geometry around the central atom?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 47
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)