Exam 5: Atomic Theory: the Nuclear Model of the Atom
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
The chemical symbol of germanium is Ge.An isotope of this element has 32 protons and 46 neutrons in the nucleus.What are the nuclear symbol,the atomic number,and the mass number of this isotope of germanium?
Nuclear symbol Atomic number Mass number
(Multiple Choice)
4.8/5  (41)
(41)
Two natural isotopes exist for silver.51.83% of the atoms have a mass of 106.90509 u,and the remaining fraction has a mass of 108.9047 u.Calculate the atomic mass of silver.
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following is the best definition of the atomic mass unit?
(Multiple Choice)
5.0/5  (41)
(41)
Consider the following pictorial representation of the postulates of Dalton's atomic theory. 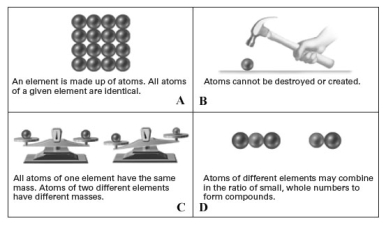 Which of the following is the best illustrates of the law of multiple proportions?
Which of the following is the best illustrates of the law of multiple proportions?
(Multiple Choice)
4.8/5  (34)
(34)
Dalton's atomic theory explained the observation that the percentage by mass of the elements in a compound is always the same,thus Dalton's atomic theory supports what Law?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 41 - 46 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)