Exam 17: Acid-Base Proton-Transferreactions
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
Consider the following image which depicts the reactants in an acid-base reaction.Atoms other than H are labeled with the element symbol. 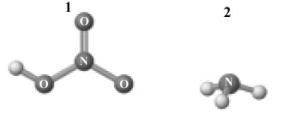 The products of this reaction are shown below.
The products of this reaction are shown below. 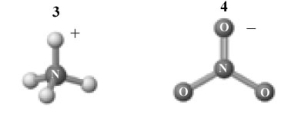 Which of the following is a correct interpretation of this reaction?
Which of the following is a correct interpretation of this reaction?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
E
Given the following relative acid strengths,starting with the weakest: HCO3- < HNO3 < HBr,what is the relative strength of each conjugate base,starting with the weakest?
Free
(Multiple Choice)
5.0/5  (48)
(48)
Correct Answer:
D
Consider a general comparison between acid-base and redox reactions.Which of the following is correct?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following statements is incorrect ?
(i)Kw = [H+][OH-] = 1.0 * 10-14 at 25°C.
(ii)Water or water solutions in which [H+] = [OH-] = 10-7 M are neutral solutions,neither acidic nor basic.
(iii)A solution in which [H+] > [OH-] is basic
Iv)A solution in which [OH- ] > [H+] is acidic
(Multiple Choice)
4.9/5  (36)
(36)
Given the following acid strengths: HF > H2S > HCN,which of the following reactions is predicted to occur from left to right as written?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following cannot act as a Brønsted-Lowry acid?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following is not capable of acting like a Brønsted-Lowry base?
(Multiple Choice)
4.8/5  (42)
(42)
Given the following relative base strengths,starting with the weakest: HSO3- < F- < C2H3O2-,what is the relative strength of each conjugate acid,starting with the weakest?
(Multiple Choice)
4.8/5  (36)
(36)
Identify the conjugate acid base pairs in the reaction H2SO4 + HNO3 H2NO3+ + HSO4-.
(Multiple Choice)
5.0/5  (45)
(45)
A solution is made by dissolving 12.50 g of NaOH,a strong base,in water to produce 2.0 liters of solution.What is the pH of this solution?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following is the correct net ionic equation for the reaction between nitrous acid and hydrogen sulfide ion?
(Multiple Choice)
4.9/5  (32)
(32)
In Brønsted-Lowry acid-base reactions,which of the following species are favored at equilibrium?
(Multiple Choice)
4.7/5  (37)
(37)
The hydroxide ion concentration of a solution is 0.00001 moles per liter.What is the hydrogen ion concentration,and is the solution acidic or basic?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 1 - 20 of 45
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)