Exam 11: Atomic Theory: the Quantum Model of the Atom
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
What is the ground state electron configuration of iron?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
How many orbitals are in the 5d sublevel?
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
C
Identify the statement among the following that combines a correct cause with a correct effect of that cause.
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
B
Which of the following statements about atomic spectra is/are correct?
(i)The discrete line spectrum of elements is evidence that electron energies are quantized
(ii)Lines in the spectrum are discrete because electron energies are restricted to certain values
(iii)Energy is emitted from atoms as electrons move from higher energy levels to lower energy levels
(iv)Electron transitions of differing energies produce different wavelengths of light.
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following statements is/are correct?
(i)Wavelength is the distance between identical parts of a wave
(ii)Frequency is the number of complete waves passing a point in a given period of time
(iii)The speed of light is equal to the product of the wavelength and frequency of an electromagnetic wave
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following is the best definition of first ionization energy?
(Multiple Choice)
4.8/5  (32)
(32)
In the third principal energy level,what is the order of energies of the sublevels,from lowest to highest?
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following is the letter representation of the electron orbital below? 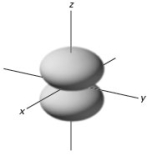
(Multiple Choice)
4.9/5  (33)
(33)
Atomic size varies with position in the periodic table primarily because of what influence(s)?
(i)Highest occupied principal energy level
(ii)Increasing number of electrons with increasing atomic number
(iii)Nuclear charge
(Multiple Choice)
4.8/5  (39)
(39)
Which general electron configuration is responsible for the family properties of noble gases?
(Multiple Choice)
4.9/5  (38)
(38)
The Bohr model of the atom assumes that which of the following quantities for the electron in a hydrogen atom is/are quantized?
(i)Speed
(ii)Direction of spin axis
(iii)Energy
(iv)Radius of its orbit
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following is/are the major flaw(s)of the Bohr model of the hydrogen atom?
(i)Hydrogen is the only atom that fits the model
(ii)Circular orbits violate the Law of Conservation of Energy
(iii)It explains atomic line spectra in terms of electron energies
(iv)It introduced the idea of quantized electron energy levels in an atom
(Multiple Choice)
4.9/5  (30)
(30)
Complete the statement with the best choice: Excited states for the hydrogen atom are states in which...
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following defines a correct relationship for electromagnetic radiation?
(Multiple Choice)
4.7/5  (41)
(41)
The order of increasing principal energy levels in an atom is related to which of the following sequences?
(Multiple Choice)
4.8/5  (40)
(40)
Consider the following periodic table. 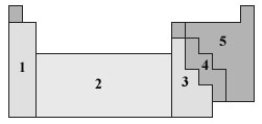 Which numbered section represents the most metallic elements?
Which numbered section represents the most metallic elements?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following lists electron sublevels in order of increasing energy?
(Multiple Choice)
4.8/5  (35)
(35)
At any instant an electron orbital may be in which occupancy state?
(i)Unoccupied
(ii)Occupied by one electron
(iii)Occupied by two electrons
(iv)Occupied by 2,6,10,or 14 electrons
(Multiple Choice)
4.8/5  (34)
(34)
Consider the following periodic table. 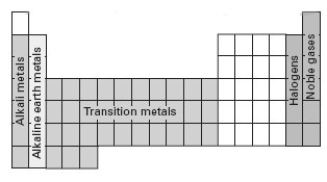 Which section represents elements whose electron configuration for the highest occupied energy level is ns2np5.
Which section represents elements whose electron configuration for the highest occupied energy level is ns2np5.
(Multiple Choice)
4.9/5  (24)
(24)
Showing 1 - 20 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)