Exam 4: Introduction to Gases
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
The pressure of the gas in a compressed gas tank is 862 kPa when the gas is at 35 °C.To what will the pressure change when the temperature drops to 7 °C?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
Which of the following correctly expresses Boyle's Law?
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
A
A plastic glove when attached to a flask gives the system a flexible volume.See the illustration below. 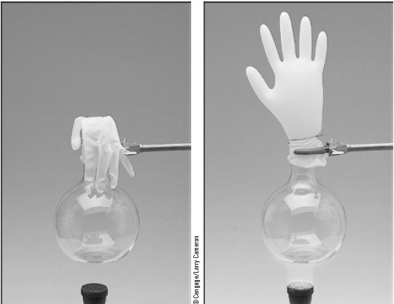 From a starting point of 257 mL at 22 °C,to what volume will the system change if the temperature rises to 137 °C due to the heating by the laboratory burner?
From a starting point of 257 mL at 22 °C,to what volume will the system change if the temperature rises to 137 °C due to the heating by the laboratory burner?
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
D
A canister holds a gas at 16.3 psi when the temperature is -15 °C.What will the pressure be when the temperature is increased to 20.°C?
(Multiple Choice)
4.7/5  (29)
(29)
A gas sample is originally at 93.9 kPa and 27 °C with a volume of 1.17 m3.What will be the resulting temperature if the pressure is adjusted to 117 kPa and the volume is reduced to 0.730 m3?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following statements are true?
(i)One standard atmosphere of pressure is defined as 760 mm Hg.
(ii)To two significant figures,one atmosphere and one bar are equal.
(iii)Gauge pressure is the pressure above atmospheric pressure.
(Multiple Choice)
4.9/5  (38)
(38)
Consider the following image showing a sample of a trapped gas in a cylinder with a movable piston. 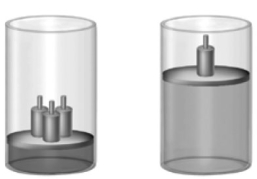 Which of the following statements is(are)illustrated by this image?
(i)Gases may be compressed.
(ii)Gases expand to fill their containers uniformly.
(iii)All gases have low density.
(iv)Gases may be mixed.
(v)A confined gas exerts constant pressure on the walls of its container uniformly in all directions.
Which of the following statements is(are)illustrated by this image?
(i)Gases may be compressed.
(ii)Gases expand to fill their containers uniformly.
(iii)All gases have low density.
(iv)Gases may be mixed.
(v)A confined gas exerts constant pressure on the walls of its container uniformly in all directions.
(Multiple Choice)
5.0/5  (39)
(39)
Consider the following graph.The data for the graph was obtained at constant temperature. 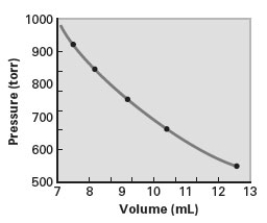 Which of the following correctly describes the relationship shown on the graph?
(i)V P
(ii)V 1/P
(iii)V = kP
(iv)V = k(1/P)
Which of the following correctly describes the relationship shown on the graph?
(i)V P
(ii)V 1/P
(iii)V = kP
(iv)V = k(1/P)
(Multiple Choice)
4.7/5  (32)
(32)
A gas has a volume of 3.77 m3 at 271 kPa.What will be its volume if the pressure is changed to 535 kPa?
(Multiple Choice)
4.8/5  (35)
(35)
A balloon is filled with a gas and has a volume of 1.79 L at 2.82 atm pressure.What will its volume be if the pressure is changed to 5.30 atm?
(Multiple Choice)
4.8/5  (28)
(28)
A gas storage tank holds a gas at 5.73 atm pressure at 22 °C.What will the pressure of the gas be when the temperature is lowered to 0 °C?
(Multiple Choice)
4.7/5  (28)
(28)
A fixed quantity of a gas is maintained at a constant temperature.The gas has a volume of 0.847 m3 at 735 torr.What is the pressure of the gas when the volume is changed to 0.905 m3?
(Multiple Choice)
4.8/5  (34)
(34)
Consider the open-end manometer shown below. 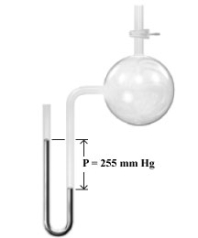 When the pressure of the atmosphere is 754 mmHg,what is the pressure of the gas sample?
When the pressure of the atmosphere is 754 mmHg,what is the pressure of the gas sample?
(Multiple Choice)
4.9/5  (35)
(35)
Examine the barometer shown below. 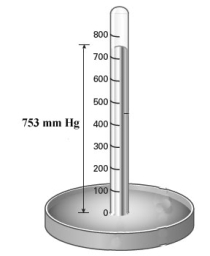 Convert the pressure reading on the barometer to atm and psi.
Convert the pressure reading on the barometer to atm and psi.
(Multiple Choice)
4.8/5  (39)
(39)
Showing 1 - 20 of 49
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)