Exam 10: Quantity Relationships in Chemical Reactions
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
H = +572 kJ for the decomposition of water by the reaction 2 H2O( 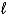 ) 2 H2(g)+ O2(g).How many grams of water can be decomposed by 5.00 *103 kJ of energy?
) 2 H2(g)+ O2(g).How many grams of water can be decomposed by 5.00 *103 kJ of energy?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
B
If the reaction N2 + 3 H2 2 NH3 is carried out using 1.40 g of N2 and 0.400 g of H2,what mass of excess reactant will remain?
Free
(Multiple Choice)
4.7/5  (43)
(43)
Correct Answer:
C
When C2H5Cl(g)is burned in oxygen,chlorine gas is produced in addition to carbon dioxide and water vapor.5145 kJ of heat are released for every four moles of C2H5Cl(g)burned.Which of the following correctly represents the thermochemical equation?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
E
How many moles of C6H12O6 are formed when 0.250 mole of CO2 is consumed in the reaction 6 CO2 + 6 H2O C6H12O6 + 6 O2?
(Multiple Choice)
4.9/5  (43)
(43)
Silver nitrate and magnesium chloride solutions are mixed.What is the Per expression that allows one to convert moles of silver nitrate to moles of magnesium chloride?
(Multiple Choice)
4.8/5  (41)
(41)
In the reaction 2 AgI + HgI2 Ag2HgI4,2.00 g of AgI and 3.50 g of HgI2 were used.What is the limiting reactant?
(Multiple Choice)
4.9/5  (38)
(38)
PCl5 can be produced by the reaction PCl3 + Cl2 PCl5.What mass of PCl3 must be used to produce 127 g of PCl5 if the percent yield is 84.8%?
(Multiple Choice)
4.9/5  (35)
(35)
In the reaction CaCN2 + 3 H2O CaCO3 + 2 NH3,how many kilograms of NH3 will be produced when 25.0 kg of CaCN2 react with excess water?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following defines the percent yield for a reaction?
(Multiple Choice)
4.9/5  (32)
(32)
Cu2HgI4 is prepared according to the equation 2 CuI + HgI2 Cu2HgI4.When 2.00 g of each reactant are used,which one is the limiting reactant?
(Multiple Choice)
4.8/5  (39)
(39)
Aqueous HCl is added to an aqueous solution of NaOH at 25 °C and the result is shown in the figure below.  Which of the following correctly characterizes this reaction?
Which of the following correctly characterizes this reaction?
(Multiple Choice)
4.9/5  (42)
(42)
In the complete combustion of C3H8O3,how many moles of carbon dioxide are produced when 23.0 g of C3H8O3 burns?
(Multiple Choice)
4.8/5  (34)
(34)
When one mole of gaseous hydrogen peroxide,H2O2,is made from hydrogen and oxygen gases,the enthalpy change is -136 kJ.Which of the following correctly represents the thermochemical equation?
(i)H2(g)+ O2(g) H2O2(g)+ 136 kJ
(ii)H2(g)+ O2(g)+ 136 kJ H2O2(g)
(iii)H2(g)+ O2(g) H2O2(g)?0?H = -136 kJ
(iv)H2(g)+ O2(g) H2O2(g)?0?H = +136 kJ
(Multiple Choice)
4.9/5  (30)
(30)
A chemical reaction resulted in 1.376 g of product,which represented a 78.5 percent yield for the reaction.What is the theoretical yield?
(Multiple Choice)
4.9/5  (38)
(38)
Consider the following thermochemical equation: CaO(s)+ H2O( 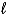 ) Ca(OH)2(s) H = -66.5 kJ Which of the following statements is/are correct?
(i)The reaction is exothermic
(ii)The reaction releases 66.5 kJ per gram of Ca(OH)2(s)formed
(iii)Alternatively,the H term could be added to the product side of the equation
) Ca(OH)2(s) H = -66.5 kJ Which of the following statements is/are correct?
(i)The reaction is exothermic
(ii)The reaction releases 66.5 kJ per gram of Ca(OH)2(s)formed
(iii)Alternatively,the H term could be added to the product side of the equation
(Multiple Choice)
4.8/5  (32)
(32)
How many moles of oxygen are consumed in the complete combustion of 1.60 moles of benzene,C6H6?
(Multiple Choice)
4.9/5  (41)
(41)
What is the theoretical yield of phosphorus triiodide when 4.50 g of phosphorus is combined with 45.0 g of iodine? The compound is made by the direct combination of the elements.
(Multiple Choice)
4.8/5  (43)
(43)
The reaction 2 Sb + 3 Cl2 2 SbCl3 has an 88.3% yield.If 4.07 g of SbCl3 is desired,what mass of chlorine should be used?
(Multiple Choice)
4.8/5  (30)
(30)
In the combustion of natural gas according to the equation CH4 + 2 O2 CO2 + 2 H2O,how many grams of water are formed during the combustion of 0.264 mole of CH4?
(Multiple Choice)
4.7/5  (42)
(42)
Showing 1 - 20 of 40
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)