Exam 13: Structure and Shape
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
Which of the following molecules is/are nonpolar?
(i)CH4
(ii)CH3Cl
(iii)CH2Cl2
(iv)CHCl3
(v)CCl4
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following has a linear electron pair geometry?
(i)IO2-
(ii)H2S
(iii)BeF2
(iv)ICl2+
(Multiple Choice)
4.7/5  (40)
(40)
Consider the following general wedge-and-dash diagram: 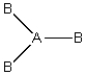 Which of the following molecules could this diagram represent?
Which of the following molecules could this diagram represent?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following has a trigonal planar molecular geometry?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 41 - 47 of 47
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)