Exam 4: The Study of Chemical Reactions
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is +0.5 kcal/mol, the Keq is ________ and the % conversion is ________.
(Multiple Choice)
4.8/5  (40)
(40)
Given an activation energy of 15 kcal/mol, use the Arrhenius equation to estimate how much faster the reaction will occur if the temperature is increased from 100°C to 120°C. R = 1.987 cal/mol∙K.
(Essay)
4.9/5  (31)
(31)
Assume the reaction A + B → C + D proceeds to equilibrium. Calculate the equilibrium concentration of D at 25°C, given that the starting concentrations of A and B are 2M and that ΔG° for the reaction is 1.0 kcal/mol. R = 1.987 cal/mol∙K.
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following reactive intermediate species maintains sp3 hybridization?
(Multiple Choice)
5.0/5  (43)
(43)
Consider the elementary step in the solvolysis of isopropyl chloride shown below and write the rate equation for this step.
(CH3)2CHCl → (CH3)2CH+ + Cl-
(Essay)
4.8/5  (36)
(36)
What is the relative reactivity of 2° vs 1° hydrogens in the free radical bromination of n-butane if the ratio of 1-bromobutane to 2-bromobutane formed is 7:93?
(Essay)
4.9/5  (32)
(32)
Predict the enthalpy (ΔH) value for the theoretical reaction below, and indicate whether it is endothermic or exothermic. The bond dissociation energy for each bond in Kcal/mol is shown below each reactant and product. 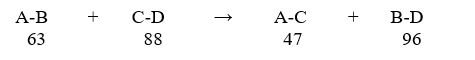
(Multiple Choice)
4.9/5  (33)
(33)
When two carbenes collide, they immediately dimerize to give ________.
(Multiple Choice)
4.8/5  (34)
(34)
Consider the conversion of X to Z through the sole intermediate Y. Given the reaction-energy diagram shown below, which step is the rate-limiting step? Explain your reasoning. 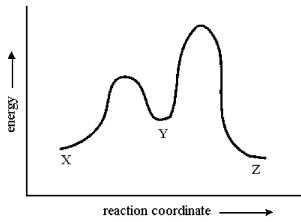
(Essay)
4.9/5  (30)
(30)
In the reaction of Cl2 with ethane and UV light, which of the following reactions would be a chain termination event(s)?
I. Cl∙ + CH3-CH3 → CH3-CH2-Cl + H∙
II. Cl∙ + CH3-CH3 → CH3-H2C∙ + HCl
III. Cl∙ + CH3-H2C∙ → CH3-CH2-Cl
IV. Cl2 + CH3-H2C∙ → CH3-CH2-Cl + Cl∙
V. Cl2 + UV light → C l∙ + Cl∙
(Multiple Choice)
4.9/5  (30)
(30)
Explain the significance of the exponential factor e-Ea/RT in the Arrhenius equation.
(Essay)
4.7/5  (31)
(31)
The Arrhenius equation mathematically models which of the following statements?
(Multiple Choice)
4.9/5  (41)
(41)
Consider the one-step conversion of F to G. Given that the reaction is endothermic by 5 kcal/mol and that the energy difference between G and the transition state for the process is 15 kcal/mol, sketch a reaction-energy diagram for this reaction. Make sure to show how the given energy differences are consistent with your sketch.
(Essay)
4.7/5  (26)
(26)
What is the name of the major monobrominated product which results when 3-methylpentane is subjected to Br2/hν conditions?
(Short Answer)
4.8/5  (44)
(44)
When acetaldehyde (CH3CHO) is deprotonated, the resulting anion is stabilized by resonance. Draw the major resonance contributing forms of this anion.
(Essay)
4.8/5  (27)
(27)
Which of the presented mechanisms would be the most energetically favorable and thus the most likely mechanism to actually occur for the following free radical chain reaction? (bond dissociation energies -- H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2
 2 HCl
2 HCl
(Multiple Choice)
4.7/5  (38)
(38)
What reactive species is produced in the initiation step of the free radical chlorination of 2,2-dimethylpropane?
(Multiple Choice)
4.7/5  (46)
(46)
How many distinct monochlorinated products can result when isobutane is subjected to free radical chlorination?
(Multiple Choice)
4.7/5  (33)
(33)
Showing 101 - 120 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)