Exam 4: The Study of Chemical Reactions
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
In the first propagation step of the free radical chlorination of methane, which of the following occurs?
(Multiple Choice)
4.8/5  (36)
(36)
The hydrogenation of acetylene to produce ethane is shown below. Is ΔS° for this reaction positive, negative, or impossible to predict? Explain your reasoning.
C2H2 (g) + 2H2 (g) → C2H6 (g)
(Essay)
4.8/5  (42)
(42)
Provide the structure of the carbene that results when diazomethane (shown below) decomposes. 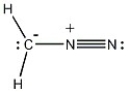
(Essay)
4.8/5  (37)
(37)
Which of the following statements correctly describes the contribution of ΔS° to ΔG°?
(Multiple Choice)
4.8/5  (43)
(43)
Consider the bond dissociation energies listed below in kcal/mol.  These data show that the carbon-bromine bond is weakest when bromine is bound to a ________.
These data show that the carbon-bromine bond is weakest when bromine is bound to a ________.
(Multiple Choice)
4.8/5  (44)
(44)
If stronger bonds are formed and weaker bonds are broken, then the reaction is ________.
(Short Answer)
4.9/5  (37)
(37)
Describe the hybridization of the cationic center and predict the CCC bond angle in (CH3)3C+.
(Essay)
4.8/5  (40)
(40)
Free radical bromination of pentane results in poor yields of 1-bromopentane, while cyclopentane can be readily brominated under similar conditions to yield bromocyclopentane. Offer an explanation.
(Essay)
4.9/5  (37)
(37)
How many distinct dichlorination products can result when isobutane is subjected to free radical chlorination?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following is true for the initiation step of a free radical chlorination reaction?
(Multiple Choice)
4.8/5  (39)
(39)
Given the chlorination of acetone shown below, choose the correct rate law. CH3COCH3 + Cl2 → CH3COCH2Cl + HCl
(Multiple Choice)
5.0/5  (33)
(33)
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is -0.5 kcal/mol, the Keq is ________ and the % conversion is ________.
(Multiple Choice)
4.9/5  (38)
(38)
________ is the minimum kinetic energy reacting molecules must possess to overcome the repulsions between their electron clouds when they collide.
(Essay)
4.8/5  (35)
(35)
Given that the theoretical reaction below was found to be second order and bimolecular, provide a rate equation for the reaction.
A-B + C-D → A-C + B-D
(Short Answer)
4.8/5  (38)
(38)
Which of the following statements is the best statement of the Hammond Postulate?
(Multiple Choice)
4.8/5  (32)
(32)
In the hydrocarbon shown below, how many tertiary hydrogens are present? 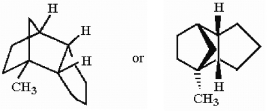
(Multiple Choice)
4.8/5  (36)
(36)
Provide the two propagation steps in the free-radical chlorination of ethane.
(Essay)
4.9/5  (31)
(31)
Showing 21 - 40 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)