Exam 4: The Study of Chemical Reactions
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Consider the reaction (CH3)3CBr + CH3CH2OH → (CH3) 3COCH2CH3 + HBr. Experimentally one finds that if the concentration of (CH3)3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
(Multiple Choice)
4.9/5  (32)
(32)
Chlorination of methane can result in a mixture of chlorinated products. What experimental conditions should be used to favor the production of chloromethane over the other chlorinated products?
(Essay)
4.9/5  (30)
(30)
Given a reaction in which reactant A is converted only to product B at 25°C, what Keq results if at equilibrium 80% of A has become B?
(Short Answer)
4.8/5  (33)
(33)
Which of the following correctly expresses the standard Gibbs free energy change of a reaction in terms of the changes in enthalpy and entropy?
(Multiple Choice)
4.9/5  (32)
(32)
When compound I, C7H16, was treated with chlorine and light it yielded 3 monochlorination products that could be separated by chromatography. Two of the products were primary alkyl halides and the other was a secondary alkyl halide. Provide a possible structure for compound I.
(Essay)
4.8/5  (33)
(33)
Explain the significance of the frequency factor A in the Arrhenius equation.
(Essay)
4.8/5  (37)
(37)
Provided the following pKa values for the two acids below, draw an energy diagram
for the acid-base reaction. Be sure to label each axis correctly. 
(Essay)
4.9/5  (29)
(29)
Which of the following reactive intermediates can best be described as both nucleophilic and strongly basic?
(Multiple Choice)
4.8/5  (35)
(35)
Write an equation to describe the initiation step in the chlorination of methane.
(Short Answer)
4.8/5  (29)
(29)
A certain free radical chlorination reaction of 2,3-dimethylbutane leads to the production of two major monochlorinated products 1-chloro-2,3-dimethylbutane and 2-chloro-2,3-dimethylbutane. When run through a Gas chromatograph, it was determined that the product distribution was 51.7% 1-chloro-2,3-dimethylbutane and 48.3% 2-chloro-2,3-dimethylbutane. If we assign the primary H's a reactivity of 1.0, what is the relative reactivity of the tertiary H's?
(Multiple Choice)
4.8/5  (40)
(40)
The chlorination of methane is characterized by a high quantum yield. Explain what this means.
(Essay)
4.9/5  (35)
(35)
Given a DG° of -8.0 kJ/mol at 25°C, calculate the corresponding K. [R = 8.314 J/K∙ mol]
(Short Answer)
4.8/5  (33)
(33)
Consider the three-step mechanism for the reaction of A through intermediates B and C to produce D shown below.
A → B Ea = 15 kcal/mol, ΔH° = 13 kcal/mol
B → C Ea = 10 kcal/mol, ΔH° = -6 kcal/mol
C → D Ea = 2 kcal/mol, ΔH° = -20 kcal/mol
Which of the three steps is rate-limiting?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is a possible termination step in the free radical chlorination of methane?
(Multiple Choice)
4.8/5  (37)
(37)
What reactive intermediate is formed when CHBr3 is treated with hydroxide or when diazomethane is irradiated?
(Multiple Choice)
4.9/5  (35)
(35)
Given the bond dissociation energies below (in kcal/mol), calculate the overall ΔH° for the following reaction:
(CH3)3CH + Br2 → (CH3)3CBr + HBr
(CH3)3C-H 91
(CH3)3C-Br 65
Br-Br 46
H-Br 88
CH3-Br 70
(Short Answer)
4.9/5  (36)
(36)
Consider the reaction: CH3CH2∙ + Br2 → CH3CH2Br + Br∙ .
Given that this reaction has an activation energy of +6 kcal/mol and a ΔH° of -22 kcal/mol, sketch a reaction-energy diagram for this reaction. Label the axes and show Ea and ΔH° on your drawing.
(Essay)
4.7/5  (39)
(39)
Within the visible spectrum, it has been experimentally determined that blue light is the most effective in initiating the chlorination of methane. What is the mechanistic significance of this observation?
(Essay)
4.9/5  (41)
(41)
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 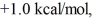 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
(Multiple Choice)
4.7/5  (38)
(38)
Showing 61 - 80 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)