Exam 4: The Study of Chemical Reactions
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
How do alkyl substituents stabilize a carbocationic center to which they are attached?
(Multiple Choice)
4.7/5  (47)
(47)
Do you expect the initiation step in the free radical chlorination of 2,2-dimethylpropane to be endo- or exothermic? Explain briefly, and comment on the sign of DH.
(Essay)
4.8/5  (36)
(36)
When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C, the relative reactivity of the 1°: 2°: 3° hydrogens is approximately 1:82:1600. Estimate the amount of each monobromination product.
(Essay)
4.8/5  (31)
(31)
Remove an H+ from the following structure to create the most reactive (least stable) carbanion. 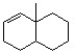
(Essay)
4.8/5  (41)
(41)
Provide the major organic product that results when 2,2,4-trimethylpentane is subjected to free radical bromination.
(Essay)
4.8/5  (33)
(33)
The following reaction occurs readily: 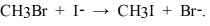 Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
(Short Answer)
4.9/5  (43)
(43)
Given that tertiary H atoms react with a chlorine radical about 5.5 times faster than primary ones, estimate the ratio of the two monochlorinated products that result when 2,3-dimethylbutane undergoes free radical chlorination.
(Essay)
4.8/5  (36)
(36)
Which of the halogens below undergoes free radical halogenation with ethane most rapidly?
(Multiple Choice)
4.8/5  (29)
(29)
What term describes the highest-energy structure in a molecular collision which leads to reaction?
(Short Answer)
4.8/5  (39)
(39)
Provide the structure of the transition state in the first propagation step of the free radical chlorination of ethane.
(Essay)
4.7/5  (42)
(42)
Given the bond dissociation energies below (in kcal/mol), estimate the ΔH° for the propagation step 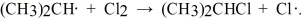 CH3CH2CH2-H 98
(CH3)2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3)2CH-Cl 80
CH3CH2CH2-H 98
(CH3)2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3)2CH-Cl 80
(Multiple Choice)
4.9/5  (35)
(35)
In an exothermic reaction, are stronger bonds broken and weaker bonds formed or are weaker bonds broken and stronger bonds formed?
(Essay)
4.8/5  (37)
(37)
Rank the free radicals (I-III) shown below in order of decreasing stability (i.e., from most stable to least stable). 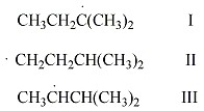
(Multiple Choice)
4.8/5  (37)
(37)
Do you expect the initiation step in the free radical chlorination of 2,2-dimethylpropane to have a positive or negative DS? Explain briefly.
(Essay)
4.9/5  (39)
(39)
What accounts for the relatively high stability of the benzyl radical?
(Essay)
4.9/5  (29)
(29)
Which of the following is true for the termination step of a free radical chlorination reaction?
(Multiple Choice)
4.7/5  (39)
(39)
Draw an energy diagram for a two step reaction where the structure of the transition state of the rate determining step most closely resembles the starting material and the overall reaction is exothermic.
(Essay)
4.8/5  (41)
(41)
If ΔG° for a given reaction at 25°C is less than zero, which of the following statements also correctly describes this reaction at this temperature?
(Multiple Choice)
4.7/5  (32)
(32)
When Br radical reacts with 1-butene (CH3CH2CH=CH2), the hydrogen atom which is preferentially abstracted is the one which produces a resonance stabilized radical. Draw the major resonance contributing forms of this radical.
(Essay)
4.8/5  (34)
(34)
Showing 41 - 60 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)