Exam 10: The Citrate Cycle
Exam 1: Principles of Biochemistry100 Questions
Exam 2: Physical Biochemistry: Energy Conversion, Water, and Membranes100 Questions
Exam 3: Nucleic Acid Structure and Function100 Questions
Exam 4: Protein Structure100 Questions
Exam 5: Methods in Protein Biochemistry100 Questions
Exam 6: Protein Function114 Questions
Exam 7: Enzyme Mechanisms106 Questions
Exam 8: Cell Signaling Systems102 Questions
Exam 9: Glycolysis: a Paradigm of Metabolic Regulation100 Questions
Exam 10: The Citrate Cycle100 Questions
Exam 11: Oxidative Phosphorylation99 Questions
Exam 12: Photosynthesis100 Questions
Exam 13: Carbohydrate Structure and Function100 Questions
Exam 14: Carbohydrate Metabolism100 Questions
Exam 15: Lipid Structure and Function100 Questions
Exam 16: Lipid Metabolism100 Questions
Exam 17: Amino Acid Metabolism100 Questions
Exam 18: Nucleotide Metabolism99 Questions
Exam 19: Metabolic Integration101 Questions
Exam 20: DNA Replication, Repair, and Recombination99 Questions
Exam 21: RNA Synthesis, Processing, and Gene Silencing100 Questions
Exam 22: Protein Synthesis, Posttranslational Modification, and Transport100 Questions
Exam 23: Gene Regulation100 Questions
Select questions type
Which molecules function as electron acceptors in the citrate cycle?
(Multiple Choice)
4.9/5  (44)
(44)
How would an increase in Ca2+ be expected to affect the pyruvate dehydrogenase reaction?
(Multiple Choice)
4.8/5  (35)
(35)
How many ATP are produced from two turns of the citrate cycle?
(Multiple Choice)
4.9/5  (30)
(30)
Predict the fate of acetyl-CoA and oxaloacetate when the citrate cycle is inhibited.
(Essay)
4.9/5  (36)
(36)
Which coenzyme in the citrate cycle is affected by arsenic?
(Multiple Choice)
4.8/5  (40)
(40)
When the citrate cycle is inhibited, which two metabolites are exported to the cytosol for fatty acid and cholesterol synthesis?
(Multiple Choice)
4.8/5  (37)
(37)
Which two enzymes in the citrate cycle are affected by arsenic poisoning?
(Multiple Choice)
5.0/5  (41)
(41)
The poison compound 1080 converts fluoroacetate to fluorocitrate. Which enzyme in the citrate cycle is inhibited by this poison?
(Multiple Choice)
4.8/5  (40)
(40)
How would an increased level of acetyl-CoA be expected to affect the pyruvate dehydrogenase reaction?
(Multiple Choice)
4.9/5  (35)
(35)
The reaction catalyzed by __________ is the most exergonic reaction in the citrate cycle.
(Multiple Choice)
4.8/5  (36)
(36)
A high concentration of which molecule would inhibit citrate synthase in the citrate cycle?
(Multiple Choice)
4.9/5  (33)
(33)
Use the figure below to answer the following questions.
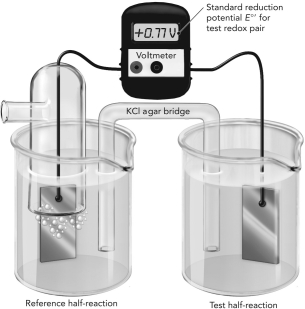 A. The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction. Write each half reaction on the figure and show the flow of electrons.
B. Do these oxidants have a higher or lower affinity for electrons compared with H+? Explain how you know this using information from the experiment.
A. The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction. Write each half reaction on the figure and show the flow of electrons.
B. Do these oxidants have a higher or lower affinity for electrons compared with H+? Explain how you know this using information from the experiment.
(Essay)
4.9/5  (28)
(28)
Which enzyme of the citrate cycle catalyzes the oxidative decarboxylation reaction that produces CO2, NADH, and succinyl-CoA?
(Multiple Choice)
4.9/5  (37)
(37)
What is the fate of oxaloacetate when it is not used in the citrate cycle?
(Multiple Choice)
4.9/5  (28)
(28)
Calculate the G ' of a redox reaction of pyruvate and hydrogen under standard conditions using the table below. 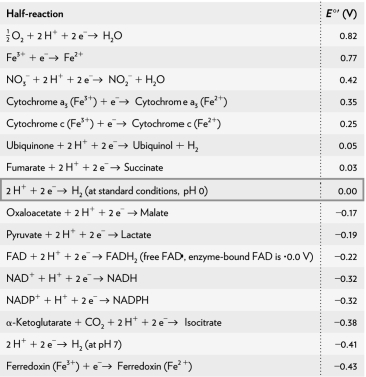
(Multiple Choice)
4.9/5  (41)
(41)
Why is the G ' of the condensation of oxaloacetate and acetyl-CoA highly favorable?
(Multiple Choice)
4.9/5  (32)
(32)
Identify the coenzyme that provides a reactive disulfide that participates in the redox reaction in the active site of pyruvate dehydrogenase.
(Multiple Choice)
4.9/5  (38)
(38)
Which anaplerotic reaction balances the input of oxaloacetate with acetyl-CoA in the citrate cycle by converting pyruvate into oxaloacetate?
(Multiple Choice)
4.9/5  (43)
(43)
Showing 41 - 60 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)