Exam 13: Chemical Kinetics
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
Given the following balanced equation, determine the rate of reaction with respect to [SO3]. 2SO2(g) + O2(g) → 2SO3(g)
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
B
What are the two key components of the frequency factor?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
A
Derive an expression for a "1/4-life" for a first-order reaction.
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
E
Derive an expression for a "1/3-life" for a first-order reaction.
(Multiple Choice)
4.9/5  (41)
(41)
How many half-lives are required for the concentration of reactant to decrease to 1.56% of its original value?
(Multiple Choice)
4.9/5  (36)
(36)
Determine the rate law and the value of k for the following reaction using the data provided: NO2(g) + O3(g) → NO3(g) + O2(g) [NO2]i (M) [O3]i (M) Initial Rate (M-1 s-1)
0)10 0.33 1.42
0)10 0.66 2.84
0)25 0.66 7.10
(Multiple Choice)
4.9/5  (33)
(33)
What data should be plotted to show that experimental concentration data fit a zeroth-order reaction?
(Multiple Choice)
4.7/5  (31)
(31)
Given the following proposed mechanism, predict the rate law for the overall reaction. A2 + 2B → 2AB (overall reaction)
Mechanism
A2 ⇌ 2A fast
A + B → AB slow
(Multiple Choice)
4.8/5  (38)
(38)
The combustion of ethylene proceeds by the reaction 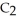
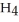 (g) + 3
(g) + 3 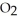 (g) → 2C
(g) → 2C 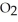 (g) +
(g) + 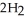 O(g) When the rate of disappearance of
O(g) When the rate of disappearance of 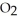 is 0.74 mol L-1
is 0.74 mol L-1 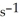 , the rate of disappearance of
, the rate of disappearance of 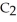
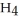 is ________
is ________ 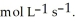
(Multiple Choice)
4.8/5  (38)
(38)
Determine the rate law and the value of k for the following reaction using the data provided: 2N2O5(g) → 4NO2(g) + O2(g) [N2O5]i (M) Initial Rate (M-1 s-1)
0)093 4.84 × 10-4
0)186 9.67 × 10-4
0)279 1.45 × 10-3
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following represents the integrated rate law for a first-order reaction?
(Multiple Choice)
4.9/5  (36)
(36)
The first-order reaction, SO2Cl2 → SO2 + Cl2, has a rate constant equal to 2.20 × 10-5 s-1 at 593 K. What percentage of the initial amount of SO2Cl2 will remain after 6.00 hours?
(Multiple Choice)
4.9/5  (35)
(35)
The aquation of tris (1,10-phenanthroline)iron(II) in acid solution takes place according to the equation: Fe(phen)32+ + 3H3O+ + 3H2O → Fe(H2O)62+ + 3phenH+.
If the activation energy, Ea, is 126 kJ mol-1 and the rate constant at 30 °C is 9.8 × 10-3 min-1, what is the rate constant at 35°C?
(Multiple Choice)
4.8/5  (36)
(36)
Explain what the exponential factor in the Arrhenius equation represents.
(Essay)
4.9/5  (39)
(39)
Given the following rate law, how does the rate of reaction change if the concentration of X is doubled? Rate = k [X]2[Y]3
(Multiple Choice)
4.9/5  (42)
(42)
For a reaction that follows the general rate law Rate = k[A]1/2[B]2, what will happen to the rate of reaction if the concentration of A and B are is increased by a factor of 4?
(Multiple Choice)
4.9/5  (34)
(34)
Nitrogen dioxide decomposes at 300 °C via a second-order process to produce nitrogen monoxide and oxygen according to the following chemical equation: 2NO2(g) → 2NO(g) + O2(g).
A sample of NO2(g) is initially placed in a 2.50 L reaction vessel at 300 °C. If the half-life and the rate constant at 300 °C are 11 seconds and 0.54 L mol-1 s-1, respectively, how many moles of NO2 were in the original sample?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 1 - 20 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)