Exam 5: Gases
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
A gas occupies 3.33 L at 2.23 bar. What is the volume at 2.50 bar?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
C
Which of the following samples has the greatest density at STP?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
D
Calculate the molecular weight of a gas that has a root mean square velocity of 269 m s-1 at 381 K.
(Multiple Choice)
4.8/5  (30)
(30)
If NO2 and NH3 are allowed to effuse through a porous membrane under identical conditions, the rate of effusion for NH3 will be ________ times that of NO2.
(Multiple Choice)
4.9/5  (39)
(39)
How many molecules of CO2 are contained in a 10.0 L tank at 7.53 bar and 485 K?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following gases has the lowest average speed at 25 °C?
(Multiple Choice)
4.7/5  (39)
(39)
A sample of 0.300 moles of nitrogen occupies 0.600 L. Under the same conditions, what number of moles occupies 1.200 L?
(Multiple Choice)
4.7/5  (28)
(28)
A sample of gas initially has a volume of 859 mL at 565 K and 2.20 bar. What pressure will the sample have if the volume changes to 268 mL while the temperature is increased to 815 K?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following compounds will behave least like an ideal gas at low temperatures?
(Multiple Choice)
4.9/5  (35)
(35)
If a scuba diver inhaled air at 3 bar pressure deep in the water and decided to swim back quickly to the surface while holding her breath, what do you expect would happen?
(Multiple Choice)
4.8/5  (25)
(25)
A gas mixture consists of N2, O2, and Ne, where the mole fraction of N2 is 0.55 and the mole fraction of Ne is 0.25. If the mixture is at STP in a 5.0 L container, how many molecules of O2 are present?
(Multiple Choice)
4.8/5  (32)
(32)
What volume (in mL) will a sample of F2 gas occupy in a syringe at 5.57 bar if the F2 has a volume of 25.0 mL at 1.22 bar?
(Multiple Choice)
4.7/5  (43)
(43)
Using the graph below, determine the gas that has the lowest density at STP. 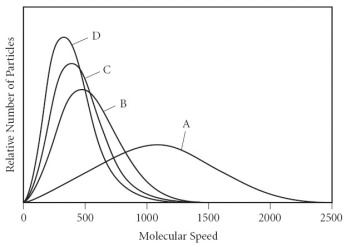
(Multiple Choice)
4.7/5  (31)
(31)
An unknown gas has a root mean square velocity of 332 m s-1 at 313 K. Identify the unknown gas.
(Multiple Choice)
5.0/5  (39)
(39)
Showing 1 - 20 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)