Exam 17: Gibbs Energy and Thermodynamics
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
How many microstates are possible in a collection of four particles that are present, with two particles each in two connected flasks?
Free
(Short Answer)
4.7/5  (32)
(32)
Correct Answer:
six or 6 or six microstates or 6 microstates
For a given reaction, ΔrH = +35.5 kJ mol-1 and ΔrS = +83.6 J K-1 mol-1. The reaction is spontaneous ________. Assume that ΔrH and ΔrS do not vary with temperature.
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
D
Which of the following statements is TRUE?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
A
Determine ΔrG° at 298 K using the following information: CaCO3(s) → CaO(s) + CO2(g) ΔrH°= +179.2 kJ mol-1; ΔrS°= +160.2 J K-1 mol-1
(Multiple Choice)
4.9/5  (41)
(41)
Given the following equation, N2O(g) + NO2(g) → 3 NO(g) ΔrG° = -23.0 kJ mol-1
Calculate ΔrG° for the following reaction:
9NO(g) → 3N2O(g) + 3NO2(g)
(Multiple Choice)
4.7/5  (40)
(40)
For the following example, what is true about ΔrH and ΔrS? H2O(l) → H2O(g)
(Multiple Choice)
5.0/5  (38)
(38)
Estimate ΔrG° for the following reaction at 449.0 K. CH2O(g) + 2H2(g) → CH4(g) + H2O(g) ΔrH°= -94.9 kJ mol-1; ΔrS°= -224.2 J K-1 mol-1
(Multiple Choice)
4.9/5  (39)
(39)
Consider the following reaction at constant pressure. Use the information here to determine the value of ?Ssurr at 355 K. Predict whether or not this reaction will be spontaneous at this temperature. 2NO(g) + O2(g) ? 2NO2(g) ?rH = -114 kJ
(Multiple Choice)
4.8/5  (35)
(35)
Place the following in order of decreasing standard molar entropy. N2O4 NO NO2
(Multiple Choice)
4.8/5  (34)
(34)
Use Hess's law to calculate ΔrG° using the following information: ClO(g) + O3(g) → Cl(g) + 2O2(g) ΔrG° = ?
2O3(g)→ 3O2(g) ΔrG° = +489.6 kJ mol-1
Cl(g) + O3(g) → ClO(g) + O2(g) ΔrG° = -34.5 kJ mol-1
(Multiple Choice)
5.0/5  (39)
(39)
Identify the compound with the lowest standard Gibbs energy of formation.
(Multiple Choice)
4.9/5  (27)
(27)
Use the following thermodynamic values to calculate Δr 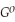 .
Δr
.
Δr
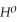 = -95 kJ
= -95 kJ  , Δr
, Δr
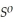 = -157 J
= -157 J 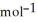 , T = 298 K
, T = 298 K
(Multiple Choice)
4.8/5  (34)
(34)
What is the name of the reaction that achieves the theoretical limits with respect to the change in Gibbs energy in thermodynamics?
(Multiple Choice)
4.8/5  (28)
(28)
Determine ΔrG° at 298 K using the following information: N2(g) + O2(g) → 2NO(g) ΔrH°= +182.6 kJ mol-1; ΔrS°= +24.8 J K-1 mol-1
(Multiple Choice)
4.7/5  (38)
(38)
Calculate ΔrS° for the following reaction. The S° for each species is shown below the reaction. 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
S°(J K-1 mol-1) 192.8 205.2 210.8 188.8
(Multiple Choice)
4.9/5  (29)
(29)
Use the following thermodynamic values to calculate Δr 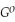 .
Δr
.
Δr
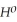 = +95 kJ
= +95 kJ 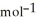 , Δr
, Δr
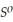 = -157 J
= -157 J 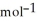 , T = 398 K
, T = 398 K
(Multiple Choice)
4.9/5  (32)
(32)
Consider a reaction that has a positive ΔrH and a positive ΔrS. Which of the following statements is TRUE?
(Multiple Choice)
5.0/5  (31)
(31)
Calculate ΔrG° at 298 K using the following information: 2HNO3(aq) + NO(g) → 3NO2(g) + H2O(l) ΔrG° = ?
ΔfG° (kJ mol-1) -110.9 87.6 51.3 -237.1
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 134
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)