Exam 6: Thermochemistry
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
Use the information provided to determine 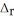 H° for the following reaction:
H° for the following reaction: 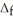 H° (kJ
H° (kJ 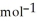 ) 3Fe2O3(s) + CO(g) → 2Fe3O4(s) + CO2(g)
) 3Fe2O3(s) + CO(g) → 2Fe3O4(s) + CO2(g) 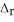 H° = ? Fe2O3(s) -824
Fe3O4(s) -1118
CO(g) -111
CO2(g) -394
H° = ? Fe2O3(s) -824
Fe3O4(s) -1118
CO(g) -111
CO2(g) -394
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
C
Given w = 0, an endothermic reaction has which of the following properties?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
C
How much energy is evolved during the formation of 98.7 g of Fe according to the reaction below?
Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(s) 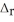 H° = -852 kJ
H° = -852 kJ
Free
(Multiple Choice)
4.8/5  (23)
(23)
Correct Answer:
A
An unknown metal alloy, mass = 26.3 g, has a temperature increase of 8.31 °C after a heat transfer of 94.0 J. Calculate the specific heat capacity of the alloy.
(Multiple Choice)
4.9/5  (28)
(28)
How much heat is absorbed when 50.00 g of C(s) reacts in the presence of excess SO2(g) to produce CS2(l) and CO(g) according to the following chemical equation?
5C(s) + 2SO2(g) → CS2(l) + 4CO(g) 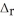 H° = 239.9 kJ
H° = 239.9 kJ
(Multiple Choice)
4.7/5  (31)
(31)
Calculate the final temperature of a 38.1 g piece of graphitic carbon, originally at 100.0 °C, placed inside a coffee cup calorimeter containing 68.0 mL of water at 24.6 °C. The specific heat capacity of water is 4.184 J g-1 °C-1, the specific heat capacity of graphitic carbon is 0.709 J g-1 °C-1, and the density of water is 1.00 g mL-1.
(Multiple Choice)
4.8/5  (41)
(41)
A sample of copper absorbs 43.6 kJ of heat, resulting in a temperature rise of 75.0 °C. Determine the mass (in kg) of the copper sample if the specific heat capacity of copper is 0.385 J g-1 °C-1.
(Multiple Choice)
4.8/5  (32)
(32)
Use the standard reaction enthalpies given below to determine 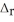 H° for the following reaction: 2S(s) + 3O2(g) → 2SO3(g)
H° for the following reaction: 2S(s) + 3O2(g) → 2SO3(g) 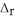 H° = ?
Given:
SO2(g) → S(s) + O2(g)
H° = ?
Given:
SO2(g) → S(s) + O2(g) 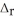 H° = +296.8 kJ
2SO2(g) + O2(g) → 2SO3(g)
H° = +296.8 kJ
2SO2(g) + O2(g) → 2SO3(g) 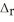 H° = -197.8 kJ
H° = -197.8 kJ
(Multiple Choice)
4.7/5  (35)
(35)
According to the following thermochemical equation, what mass of HF (in g) must react to produce 345 kJ of energy? Assume excess SiO2. SiO2(s) + 4HF(g) → SiF4(g) + 2H2O(l) 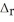 H° = -184 kJ
H° = -184 kJ
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the change in internal energy (ΔU) for a system that is giving off 25.0 kJ of heat and is changing from 12.00 L to 6.00 L in volume at 1.50 bar. (Remember that 100 J = 1 L bar)
(Multiple Choice)
4.8/5  (35)
(35)
Match the following.
-energy associated with the motion of an object
(Multiple Choice)
4.8/5  (38)
(38)
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemical equation shown below. When 0.0 15 mol of Na is added to 100.00 g of water, the temperature of the resulting solution rises from 25.00 °C to 31.45 °C. If the specific heat of the solution is 4.18 J 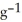
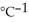 , calculate ΔH for the reaction, as written. 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
, calculate ΔH for the reaction, as written. 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) 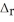 H= ?
H= ?
(Multiple Choice)
4.7/5  (38)
(38)
A piece of uranium with a mass of 12.1 g and an initial temperature of 60.2 °C was placed inside a coffee cup calorimeter. The temperature of the water inside the calorimeter increased from 22.8 °C to 23.9 °C. The specific heat capacity of water is 4.184 J g-1 °C-1 and the specific heat capacity of uranium is 0.116 J g-1 °C-1. Calculate the mass of water contained inside the calorimeter.
(Multiple Choice)
4.9/5  (33)
(33)
The specific heat capacity of liquid water is 4.184 J 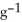
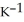 . How many joules of heat are needed to raise the temperature of 7.00 g of water from 33.0 °C to 76.0 °C?
. How many joules of heat are needed to raise the temperature of 7.00 g of water from 33.0 °C to 76.0 °C?
(Multiple Choice)
4.7/5  (32)
(32)
A balloon is inflated from 0.0100 L to 0.500 L against an external pressure of 10.00 bar. How much work is done in joules? (100 J = 1 L bar)
(Multiple Choice)
4.9/5  (36)
(36)
Use the information provided to determine 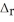 H° for the following reaction:
H° for the following reaction: 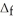 H°(kJ
H°(kJ 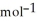 ) CH4(g) + 4 Cl2(g) → CCl4(g) + 4 HCl(g)
) CH4(g) + 4 Cl2(g) → CCl4(g) + 4 HCl(g) 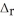 H° = ? CH4(g) -75
CCl4(g) -96
HCl(g) -92
H° = ? CH4(g) -75
CCl4(g) -96
HCl(g) -92
(Multiple Choice)
4.7/5  (29)
(29)
Showing 1 - 20 of 161
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)