Exam 14: Chemical Equilibrium
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
For which of the following reactions will ∆n = 1?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
C
The decomposition of ammonia is 2NH3(g) → N2(g) + 3H2(g). If Kp is 1.5 × 103 bar2 at 400 °C, what is the partial pressure of ammonia at equilibrium when the partial pressure of N2(g) is 0. 10 bar and that of H2(g) is 0.15 bar?
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
B
Give the direction of the reaction if K >> 1.
Free
(Multiple Choice)
4.9/5  (46)
(46)
Correct Answer:
A
The following reaction is exothermic. Which change will shift the equilibrium to the left? 2SO2(g) + O2(g) ⇌ 2SO3(g)
(Multiple Choice)
4.8/5  (39)
(39)
The equilibrium constant is given for one of two reactions below. Determine the value of the missing equilibrium constant. 2SO2(g) + O2(g) ⇌ 2SO3(g) K = 1.7 × 106
SO3(g) ⇌ 1/2 O2(g) + SO2(g) K = ?
(Multiple Choice)
5.0/5  (31)
(31)
The equilibrium constant is given for one of two reactions below. Determine the value of the missing equilibrium constant. H2(g) + Br2(g) ⇌ 2HBr(g) K = 3.8 × 104
4HBr(g) ⇌ 2H2(g) + 2Br2(g) K = ?
(Multiple Choice)
4.7/5  (33)
(33)
Consider the following reaction at equilibrium. What effect will increasing the temperature have on the system? Fe3O4(s) + CO(g) ⇌ 3FeO(s) + CO2(g) ΔrH°= +35.9 kJ
(Multiple Choice)
4.8/5  (28)
(28)
Determine the value of Kp for the following reaction if the equilibrium concentrations are as follows: P(CO)eq = 6.8 × 10-11 atm, P(O2)eq = 1.3 × 10-3 atm, P(CO2)eq = 0.041 atm. 2 CO(g) + O2(g) ⇌ 2 CO2(g)
(Multiple Choice)
4.7/5  (40)
(40)
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of H2O(g). C2H4(g) + H2O(g) ⇌ C2H5OH(g) Kc = 9.0 × 103 M-1
[C2H4]eq = 0.015 mol L-1 [C2H5OH]eq = 1.69 mol L-1
(Multiple Choice)
4.8/5  (32)
(32)
The reaction below has a Kc value of 61 M-2. What is the value of Kp for this reaction at 500 K? N2(g) + 3H2(g) ⇌ 2NH3(g)
(Multiple Choice)
4.9/5  (37)
(37)
Consider the following reaction at equilibrium. What effect will increasing the pressure of the reaction mixture have on the system? 2H2S(g) + 3O2(g) ⇌ 2H2O(g) + 2SO2(g)
(Multiple Choice)
4.9/5  (44)
(44)
Phosphorus trichloride and phosphorus pentachloride equilibrate in the presence of molecular chlorine according to the following reaction: 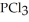 (g) +
(g) + 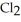 (g) →
(g) → 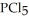 (g) An equilibrium mixture at 450 K contains
(g) An equilibrium mixture at 450 K contains 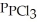 = 0.124 bar,
= 0.124 bar, 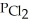 = 0.157 bar, and
= 0.157 bar, and 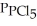 = 1.30 bar. What is the value of Kp at this temperature?
= 1.30 bar. What is the value of Kp at this temperature?
(Multiple Choice)
4.8/5  (35)
(35)
Consider the following reaction and its equilibrium constant: I2(g) ⇌ 2I(g) Kp = 0.209 atm
A reaction mixture contains 4.0 atm I2 and 0.5 atm I. Which of the following statements is TRUE concerning this system?
(Multiple Choice)
4.8/5  (38)
(38)
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [N2]eq = 1.5 mol L-1, [H2]eq = 1.1 mol L-1, [NH3]eq = 0.47 mol L-1. N2(g) + 3H2(g) ⇌ 2NH3(g)
(Multiple Choice)
4.9/5  (35)
(35)
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [H2]eq = 0.14 mol L-1, [I2]eq = 0.39 mol L-1, [HI]eq = 1.6 mol L-1. H2(g) + I2(g) ⇌ 2HI(g)
(Multiple Choice)
4.7/5  (33)
(33)
For the following reaction, what is △n required in the conversion of Kc to Kp? 2Na(s) + 2H2O(l) ⇌ 2NaOH(aq) + H2(g)
(Multiple Choice)
4.9/5  (28)
(28)
Express the equilibrium constant for the following reaction: 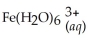 + 6CN-(aq) ⇌
+ 6CN-(aq) ⇌ 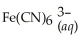 + 6H2O(l)
+ 6H2O(l)
(Multiple Choice)
4.7/5  (33)
(33)
Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the equation: 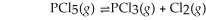 At 250° 0. 125 mol L-1 PCl5 is added to the flask. If Kc = 1.80 mol L-1, what are the equilibrium concentrations of each gas?
At 250° 0. 125 mol L-1 PCl5 is added to the flask. If Kc = 1.80 mol L-1, what are the equilibrium concentrations of each gas?
(Multiple Choice)
4.9/5  (38)
(38)
At a certain temperature, nitogen and hydrogen react to form ammonia: N2(g) + 3 H2(g) ⇌ 2 NH3(g)
When initial amounts of N2, H2, and NH3 are mixed, the concentration of NH3 increases. Which statement below is TRUE?
(Multiple Choice)
4.8/5  (25)
(25)
Showing 1 - 20 of 150
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)