Exam 3: Ionic and Covalent Compounds
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Which of the following statements about the adenosine receptor is NOT true?
(Multiple Choice)
4.7/5  (41)
(41)
Below are two charged particles.What will they do when in close proximity to one another? 
(Multiple Choice)
4.8/5  (30)
(30)
"Laughing gas" has the formula N2O.Which of the following is the best name for this compound?
(Multiple Choice)
4.9/5  (38)
(38)
Oxygen is a _______ and therefore _______ when it forms an ion.
(Multiple Choice)
4.9/5  (31)
(31)
What is the ionic formula for the ionic compound composed of calcium and chloride ions?
(Multiple Choice)
4.8/5  (32)
(32)
Below is an ion of magnesium.To make this ion, the atom that it came from had to _________. 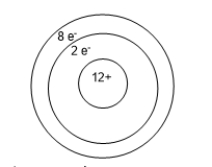
(Multiple Choice)
4.9/5  (25)
(25)
Which of the following ions does NOT have the same number of valence electrons as neon?
(Multiple Choice)
4.9/5  (41)
(41)
What is the name of the ionic compound that is composed of calcium and chlorine?
(Multiple Choice)
4.9/5  (38)
(38)
What is the charge on each chloride ion in an ionic compound composed of calcium and chlorine?
(Multiple Choice)
4.7/5  (38)
(38)
______ is a waste product excreted by the kidneys.It is a product of the breakdown of proteins.
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following elements might have an expanded octet in a covalent molecule?
(Multiple Choice)
4.8/5  (37)
(37)
Which is the BEST Lewis structure for a molecule with the formula C2H3Cl? 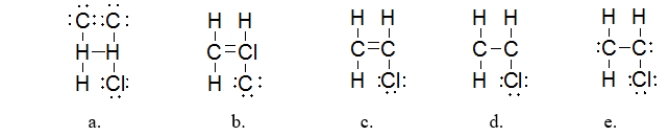
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following Lewis dot structures best represents the chlorine atom? 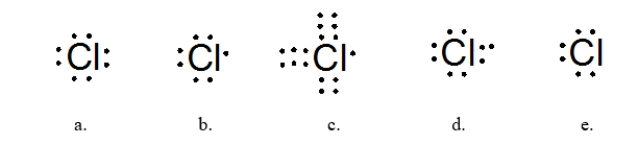
(Multiple Choice)
4.8/5  (31)
(31)
Vinegar (C2H4O2)and baking soda (NaHCO3)are combined together and the resulting mixture bubbles, releasing a gas.This gas is a(n)
(Multiple Choice)
4.9/5  (36)
(36)
Showing 61 - 80 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)