Exam 31: Nuclear Physics and Radioactivity
Exam 1: Introduction and Mathematical Concepts67 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum65 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena51 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits99 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Reflection of Light: Mirrors42 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity62 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom73 Questions
Exam 31: Nuclear Physics and Radioactivity33 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles43 Questions
Select questions type
How much energy is required to remove a neutron (mn = 1.008 665 u) from 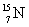 that has an atomic mass of 15.000 108 u to make
that has an atomic mass of 15.000 108 u to make 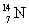 that has an atomic mass of 14.003 074 u? Note: The energy equivalent of the atomic mass unit is 931.5 MeV.
that has an atomic mass of 14.003 074 u? Note: The energy equivalent of the atomic mass unit is 931.5 MeV.
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
C
Determine the amount of energy released in the following alpha decay process: 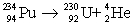 The relevant atomic masses are
The relevant atomic masses are 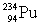 = 234.043 299 u,
= 234.043 299 u, 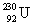 =230.033 937 u, and
=230.033 937 u, and 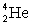 = 4.002 603 u.
= 4.002 603 u.
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
A
The activity of a carbon-14 sample is 0.1 Ci.If this sample is burned, what is the activity of the resulting CO2?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
B
Determine the activity of 6.0 × 1012 atoms of Rn-220 that has a half-life of 56 s.
(Multiple Choice)
4.7/5  (43)
(43)
This question refers to the figure shown.Which one of the following concepts explains why heavy nuclei do not follow the N = Z line (or trend) in the figure? 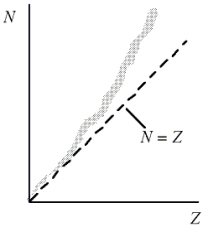
(Multiple Choice)
4.9/5  (41)
(41)
The half-life of a particular isotope of iodine is 8.0 days.How much of a 10.0-g sample of this isotope will remain after 30 days?
(Multiple Choice)
5.0/5  (31)
(31)
The binding energy of an isotope of chlorine is 298 MeV.What is the mass defect of this chlorine nucleus in atomic mass units?
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following statements concerning stable nuclei is true?
(Multiple Choice)
4.9/5  (31)
(31)
The half-life a particular isotope of barium is 12 s.What is the activity of a 1.0 × 10-6 kg sample of this isotope?
(Multiple Choice)
4.8/5  (29)
(29)
An isotope of krypton has a half-life of 3 minutes.A sample of this isotope produces 1000 counts per minute in a Geiger counter.Determine the number of counts per minute produced after 15 minutes.
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following statements is true concerning the radioisotope carbon-14 that is used in carbon dating?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following is not an assumption involved in the expression: r = (1.2 × 10-15 m)A1/3?
(Multiple Choice)
4.9/5  (31)
(31)
A sample contains 1000 nuclei of a radioactive isotope of barium.Exactly sixty seconds later, 970 nuclei in the sample have decayed.Determine the half-life of this isotope.
(Multiple Choice)
4.8/5  (31)
(31)
If the uranium nucleus is at rest before its decay, which one of the following statements is true concerning the final nuclei?
(Multiple Choice)
4.9/5  (29)
(29)
Which one of the following quantities is not a conserved quantity according to the laws of physics?
(Multiple Choice)
4.8/5  (38)
(38)
Determine the amount of energy released in this decay.Use the following atomic masses: 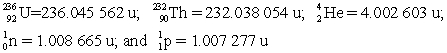 Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
(Multiple Choice)
4.9/5  (30)
(30)
The same activity is measured for two different isotope samples.One sample contains 0.0450 kg of 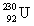 (atomic mass = 230.033 937 u, t1/2 = 20.8 days).The second sample contains an unknown amount of
(atomic mass = 230.033 937 u, t1/2 = 20.8 days).The second sample contains an unknown amount of 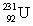 (atomic mass = 231.036 264 u, t1/2 = 4.3 days).What is the mass of the second sample?
(atomic mass = 231.036 264 u, t1/2 = 4.3 days).What is the mass of the second sample?
(Multiple Choice)
4.7/5  (46)
(46)
In a certain decay process, an excited neon atom emits a -ray that has an energy of 1.630 MeV.The neon atom in the ground state has a mass of 19.992 435 u.What is the mass of the excited neon atom?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 33
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)