Exam 30: The Nature of the Atom
Exam 1: Introduction and Mathematical Concepts67 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum65 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena51 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits99 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Reflection of Light: Mirrors42 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity62 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom73 Questions
Exam 31: Nuclear Physics and Radioactivity33 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles43 Questions
Select questions type
A neutral atom has the following electronic configuration: 1s2 2s2 2p6 3s2 3p5
-How many electrons are in the M shell?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
D
A pulsed laser has an average output power of 4.0 W.Each pulse consists of light at wavelength 5.0 × 10-7 m and has a 25 ms duration.How many photons are emitted in a single pulse?
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
B
The ground state electronic configuration of a neon atom is 1s2 2s2 2p6.How many of these electrons have magnetic quantum number ml = 0?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
C
Consider the following list of electron configurations:
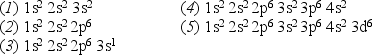 -Which one of the above configurations represents a transition element?
-Which one of the above configurations represents a transition element?
(Multiple Choice)
4.9/5  (34)
(34)
Complete the following statement: An individual copper atom emits electromagnetic radiation with wavelengths that are
(Multiple Choice)
4.8/5  (41)
(41)
An argon-ion laser emits a blue-green beam of light with a wavelength of 514.5 nm in a vacuum.What is the difference in energy in joules between the two energy states for the atomic transition that produces this light?
(Multiple Choice)
4.8/5  (41)
(41)
Which one of the following will result in an electron transition from the n = 4 level to the n = 7 level in a hydrogen atom?
(Multiple Choice)
4.9/5  (35)
(35)
The principle quantum number for the electron in a hydrogen atom is n = 5.According to the quantum mechanical picture of the atom, what is the maximum possible value for the magnitude of the z-component of the angular momentum of the electron?
(Multiple Choice)
4.7/5  (30)
(30)
The kinetic energy of the ground state electron in hydrogen is +13.6 eV.What is its potential energy?
(Multiple Choice)
5.0/5  (38)
(38)
Which transition will occur when a hydrogen atom is irradiated with radiation of frequency 1.60 × 1014 Hz?
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the following factors best explains why the six electrons of a carbon atom are not all in the 1s state?
(Multiple Choice)
4.9/5  (39)
(39)
Which one of the following statements is the assumption that Niels Bohr made about the angular momentum of the electron in the hydrogen atom?
(Multiple Choice)
4.8/5  (37)
(37)
An atom will emit photons when one of its electrons goes from
(Multiple Choice)
4.8/5  (39)
(39)
To which model of atomic structure does the Pauli exclusion principle apply?
(Multiple Choice)
4.7/5  (37)
(37)
The electron in a hydrogen atom is in the n = 3 state.What is(are) the possible value(s) for an emitted photon?
(Multiple Choice)
4.9/5  (37)
(37)
Electrons have been removed from a lithium atom (Z = 3) until only one remains.Determine the energy of the photon that can be emitted if the remaining electron is in the n = 2 level.
(Multiple Choice)
4.8/5  (42)
(42)
Consider the following list of electron configurations:
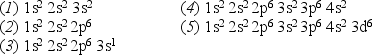 -Which one of the following statements concerning the cutoff wavelength typically exhibited in X-ray spectra is true?
-Which one of the following statements concerning the cutoff wavelength typically exhibited in X-ray spectra is true?
(Multiple Choice)
4.8/5  (32)
(32)
Consider the following list of electron configurations:
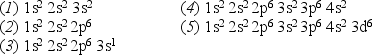 -Which one of the above configurations represents an excited state of a neutral atom?
-Which one of the above configurations represents an excited state of a neutral atom?
(Multiple Choice)
4.8/5  (27)
(27)
Consider the following list of electron configurations:
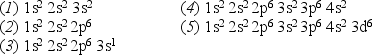 -Which electron energy will produce the largest cutoff wavelength for X-ray production from a nickel (Z = 28) surface?
-Which electron energy will produce the largest cutoff wavelength for X-ray production from a nickel (Z = 28) surface?
(Multiple Choice)
4.8/5  (33)
(33)
Electrons in an X-ray tube are accelerated through a potential difference of 40 kV.The electrons then strike a zirconium (Z = 40) target.Determine the cutoff frequency for X-ray production.
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)