Exam 20: The First Law of Thermodynamics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
If 25 kg of ice at 0°C is combined with 4 kg of steam at 100°C, what will be the final equilibrium temperature (in °C) of the system?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
B
Determine the work done by 5 moles of an ideal gas that is kept at 100°C in an expansion from 1 liter to 5 liters.
Free
(Multiple Choice)
4.8/5  (26)
(26)
Correct Answer:
A
A gas expands as shown in the graph. If the heat taken in during this process is 1.02 × 106 J and 1 atm = 1.01 × 105 N/m2, the change in internal energy of the gas (in J) is 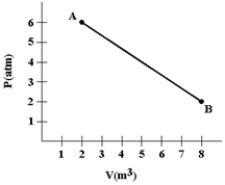
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
B
Five moles of an ideal gas expands isothermally at 100°C to five times its initial volume. Find the heat flow into the system.
(Multiple Choice)
4.8/5  (40)
(40)
The R-value of an insulating material is the thickness of the material divided by its thermal conductivity. When an insulating material consists of three layers with R-values R1, R2 and R3, the overall R-value of the insulation is given by
(Multiple Choice)
4.8/5  (32)
(32)
For an astronaut working outside a spaceship, the greatest loss of heat would occur by means of
(Multiple Choice)
4.8/5  (38)
(38)
A 300-g glass thermometer initially at 25°C is put into 200 cm3 of hot water at 95°C. Find the final temperature (in °C) of the thermometer, assuming no heat flows to the surroundings. (The specific heat of glass is 0.2 cal/g⋅°C.)
(Multiple Choice)
4.9/5  (32)
(32)
Water at room temperature, 20°C, is pumped into a reactor core where it is converted to steam at 200°C. How much heat (in J) is transferred to each kilogram of water in this process? (csteam = 2 010 J/kg⋅°C; Lsteam = 2.26 × 103 J/g; 1 cal = 4.186 J.)
(Multiple Choice)
4.7/5  (42)
(42)
Gas in a container increases its pressure from 1 atm to 3 atm while keeping its volume constant. Find the work done (in J) by the gas if the volume is 5 liters.
(Multiple Choice)
5.0/5  (33)
(33)
A wall is constructed of a 2 inch layer of fiberglass board (R = 8) and six inches of fiberglass batting (R = 19). If the temperature on the outside surface of the fiberglass board is 50°F and the temperature on the inside surface of the fiberglass batting is 68°F, what is the temperature (in °F) at the interface? (The units of R are ft2⋅°F⋅h/BTU.)
(Multiple Choice)
4.8/5  (33)
(33)
How much water at 20°C is needed to melt 1 kilogram of solid mercury at its melting point of −39°C? (The heat of fusion of mercury is 2.8 cal/gram).
(Short Answer)
4.8/5  (35)
(35)
An 8 000-kg aluminum flagpole 100-m high is heated by the sun from a temperature of 10°C to 20°C. Find the increase in internal energy (in J) of the aluminum. (The coefficient of linear expansion is 24 × 10−6 (°C)−1, the density is 2.7 × 103 kg/m3, and the specific heat of aluminum is 0.215 cal/g⋅°C.)
(Multiple Choice)
4.9/5  (31)
(31)
In which process will the internal energy of the system NOT change?
(Multiple Choice)
4.9/5  (38)
(38)
A block of material of mass m and specific heat c falls from height h and reaches speed v just before striking the ground. Its temperature is measured immediately after it strikes the ground. If we ignore any change in temperature owing to interaction with the air, the change in temperature of the block of material is
(Multiple Choice)
4.8/5  (29)
(29)
Duff states that equal masses of all substances have equal changes in internal energy when they have equal changes in temperature. Javan states that the change in internal energy is equal to a constant times the change in temperature for every ΔT, no matter how large or how small ΔT is, but that the constant is different for different substances. Which one, if either, is correct?
(Multiple Choice)
4.9/5  (24)
(24)
How many calories of heat are required to raise the temperature of 4 kg of water from 50°F to the boiling point?
(Multiple Choice)
4.9/5  (38)
(38)
Showing 1 - 20 of 61
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)