Exam 42: Atomic Physics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
The allowed values of 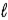 for the n = 3 shell in a Li2+ ion are
for the n = 3 shell in a Li2+ ion are
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
C
For the following allowed transitions, which photon would have the largest wavelength when an electron "jumps" from one energy level, characterized by the quantum number n, to another?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
B
The probability density for the 1s state is given by |Ψ1s|2. The probability of finding the particle somewhere in space is
Free
(Multiple Choice)
4.9/5  (24)
(24)
Correct Answer:
A
When electrons fill a subshell in which the orbitals have equal energy, the order in which the orbitals are filled is such that
(Multiple Choice)
4.9/5  (32)
(32)
The number of electrons in the n = 4, 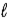 = 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4,
= 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4, 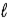 =2 subshell in barium (Z = 56).
=2 subshell in barium (Z = 56).
(Multiple Choice)
4.9/5  (41)
(41)
When using the Pauli Exclusion Principle, we assume the particle's spin angular momentum is of magnitude
(Multiple Choice)
4.8/5  (31)
(31)
Of the following states, 5s, 3p, 4f, 5p, 4g, 3d, and 2p, the one which is NOT allowed is
(Multiple Choice)
4.8/5  (37)
(37)
In 1921, Stern and Gerlach performed an experiment that first demonstrated
(Multiple Choice)
4.8/5  (28)
(28)
What wavelength (in μm) is associated with the Paschen series for n = 4?
(RH = 1.097 × 107 m−1)
(Multiple Choice)
4.8/5  (36)
(36)
In an atom that has an electron in a sub-shell for which 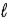 = 4, with respect to the magnetic field vector
= 4, with respect to the magnetic field vector  the magnetic moment vector
the magnetic moment vector 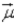 of the electron is allowed to be oriented in
of the electron is allowed to be oriented in
(Multiple Choice)
4.8/5  (34)
(34)
An electron is moving at a speed of 2.1 × 106 m/s in the first Bohr orbit. Determine its de Broglie wavelength.
(Multiple Choice)
4.9/5  (28)
(28)
In terms of a0, where a0 = 0.0529 nm, the radii of the allowed orbits in the Bohr model of the hydrogen atom are given by rn =
(Multiple Choice)
4.9/5  (38)
(38)
Zeke says that the magnitude of the orbital angular momentum in the hydrogen atom has the value L = 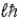 . Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector
. Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector  is
is 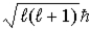 . Which one, if either, is correct, and why?
. Which one, if either, is correct, and why?
(Multiple Choice)
4.9/5  (39)
(39)
What is the difference in frequency for spectral lines emitted by hydrogen for transitions from the n = 16 level to the n = 2 level and transitions from the n = 15 level to the n = 2 level? (RH = 1.097 × 107 m−1.)
(Multiple Choice)
4.7/5  (36)
(36)
What angle does the orbital angular momentum make with the z axis of a hydrogen atom in the state n = 3, 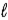 = 2,
= 2, 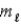 = −1?
= −1?
(Multiple Choice)
4.7/5  (33)
(33)
A hydrogen atom in the 4f state has a total angular momentum (in terms of 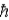 ) of magnitude
) of magnitude
(Multiple Choice)
5.0/5  (36)
(36)
In a shell of the hydrogen atom with n = 3, the permitted values of the orbital magnetic quantum number 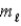 are
are
(Multiple Choice)
4.9/5  (36)
(36)
Showing 1 - 20 of 59
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)