Exam 44: Nuclear Structure
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
When a nucleus at rest spontaneously splits into fragments of mass m1 and m2, the ratio of the momentum of m1 to the momentum of m2 is
Free
(Multiple Choice)
5.0/5  (30)
(30)
Correct Answer:
C
The ratio of the radius of a classical electron (re = kee2/mec2 = 2.8 × 10−15 m) to the radius of a 4He nucleus (r = r0A1/3) is
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
C
A radioactive sample with decay rate R and decay energy Q has power output
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
B
Find the binding energy per nucleon (in MeV/nucleon) of carbon-12. Assume:
MC = 12.000 000 u
M p = 1.007 825 u
M n = 1.008 665 u
U = 1.66 × 10−27 kg
(Multiple Choice)
4.8/5  (29)
(29)
The radiocarbon content of 14C decreases after the death of a living system with a half-life of 5730 y. If an archaeologist working a dig finds an ancient firepit containing some partially consumed firewood and the wood contains only 12.5 percent of the 14C content of an equal carbon sample from a present-day tree, what is the age of the ancient site?
(Short Answer)
4.8/5  (33)
(33)
The isotope, tritium, has a half-life of 12.3 years. Assume we have 10 kg of the substance. What will be the disintegration constant (in s−1)?
(Multiple Choice)
4.7/5  (27)
(27)
The mass of 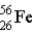 is 55.9349 u and the mass of
is 55.9349 u and the mass of 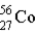 is 55.939 9 u. Which isobar decays into the other, and by what 2 possible processes?
is 55.939 9 u. Which isobar decays into the other, and by what 2 possible processes?
(Essay)
4.8/5  (38)
(38)
An alpha particle is emitted from a radioactive source with an energy of 5 MeV. How fast is it moving (in m/s)? (m = 4.002 603 u, 1 u = 1.66 × 10−27 kg.)
(Multiple Choice)
4.9/5  (32)
(32)
In neutron capture by an atomic nucleus, the mass number of the nucleus changes by
(Multiple Choice)
4.9/5  (48)
(48)
A pure sample of 226Ra contains 2.0 × 1014 atoms of the isotope. If the half-life of 226Ra = 1.6 × 103 years, what is the decay rate of this sample? (1 Ci = 3.7 × 1010 decays/s)
(Short Answer)
4.7/5  (31)
(31)
Naturally radioactive nuclei can decay spontaneously by emitting the following particles:
(Multiple Choice)
4.8/5  (32)
(32)
Approximately how fast is an ion of helium moving if it is in a plasma with a temperature of 108 K? m(He) = 4.002 603 u and u = 1.66 × 10−27 kg.
(Multiple Choice)
4.8/5  (36)
(36)
It is often possible to use the atomic masses when calculating the binding energy of a nucleus. The reason for this is
(Multiple Choice)
4.8/5  (34)
(34)
In nuclear magnetic resonance, nuclei absorb energy when flipping between nuclear
(Multiple Choice)
4.8/5  (38)
(38)
In neutron capture by an atomic nucleus, the atomic number changes by
(Multiple Choice)
4.9/5  (28)
(28)
What is the average kinetic energy (in keV) of an ion that has a temperature of 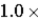 108 K?
108 K?
(Multiple Choice)
4.8/5  (43)
(43)
Find the unknown atomic number and mass number respectively, for the following reaction 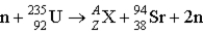
(Multiple Choice)
4.9/5  (38)
(38)
How fast must two deuterium atoms be moving so they can overcome the Coulomb force of repulsion, and attain the necessary 10−14 m for fusion? (m( 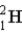 ) = 2.014 1 u)
) = 2.014 1 u)
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)