Exam 1: Matter, Energy, and Measurement
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
Given the calculation: 1.987 × 6.02 = ?, what is the answer reported to the correct number of significant figures?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
C
Which of the following is true of ice, water, and steam?
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
C
How many kilometers (km) are there is 1 megameter (Mm)?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
C
Iron has a density of 7.874 g/cm3. What is the volume of a block of iron that weighs 15.321 g?
(Multiple Choice)
4.8/5  (39)
(39)
In Europe, the areas of apartments are typically listed in square meters (m2). If the area of an American apartment is 1.6 × 103 ft2, a Paris apartment with which of the following areas has roughly the same area as the New York apartment? [1 meter = 1.094 yards]
(Multiple Choice)
4.8/5  (34)
(34)
Consider the separatory funnel shown below; it contains two liquids. Water is placed in the funnel along with one of the following liquids: diethyl ether, mineral oil, or dichloromethane. The funnel is then opened, and the bottom layer is drained into a beaker. For which combination would the water end up in the beaker? Density values are given in parentheses. 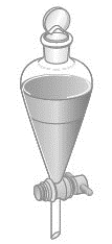
(Multiple Choice)
5.0/5  (38)
(38)
Given the calculation: 5.63 + 2.302 + 3.1 = ?, what is the answer reported to the correct number of significant figures?
(Multiple Choice)
4.8/5  (30)
(30)
A studio apartment in Paris has an area of 81.0 square meters. A New York apartment with which of the following areas has the roughly the same area as the Paris apartment? [1 meter = 1.094 yards]
(Multiple Choice)
4.8/5  (37)
(37)
In determining the density of a liquid, the following measurement was made. How many significant figures are shown in this measurement? 
(Multiple Choice)
4.7/5  (34)
(34)
An object weighs 70.7 kg. What is the weight of this object in the English system? [1 pound = 453.6 grams]
(Multiple Choice)
4.9/5  (24)
(24)
Xenon is a gas found in some automobile headlights. The density of xenon at room temperature and pressure is 5.37 g/L. What is the mass, in pounds, of 1.00 quart of xenon? [1 liter = 1.057 quart; 1 pound = 453.6 grams]
(Multiple Choice)
4.9/5  (34)
(34)
A jeweler offers a pure gold coin of 2 g to a customer. What is the volume of the coin? (Take the density of gold to be 19.3 g/cm3.)
(Multiple Choice)
4.9/5  (32)
(32)
The dimensions of a piece of wood are 3.4 meters x 10 cm x 175 mm. What is the volume of this piece of wood?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is the largest unit of mass among the given units?
(Multiple Choice)
4.7/5  (28)
(28)
An intern made an error and gave a patient a dose of 600 μg rather than 60 mg of a drug. Which of the following is true?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 143
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)