Exam 4: Chemical Reactions
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
O2 can be produced by the reaction 2KClO3(s) → 2KCl(s) + 3O2(g). In one experiment, 0.312 g of O2 was formed. How much KCl was produced?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
A
To which of the following is the term molecular weight applicable?
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
B
How many calories are required to heat 520 g of copper from 30°C to 800°C? The specific heat of copper is 0.092 cal/g·°C.
(Multiple Choice)
4.9/5  (42)
(42)
The synthesis of a certain drug requires four steps. The first three steps were relatively easy with percent yields of 97.1%, 94.3%, and 92.8%, respectively. However, the last step was very difficult, with a percent yield of 72.7%. What was the overall percent yield?
(Multiple Choice)
4.9/5  (34)
(34)
Lime, CaO, is produced by the reaction CaCO3(s) → CaO(s) + CO2(g). What weight of CaO is obtained by the decomposition of 38.7 g of CaCO3?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following statements is true of the balanced equation 3H2(g) + N2(g) → 2NH3(g)?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following represents the correct set of spectator ions in the equation Cu2+(aq) + 2Cl-(aq) + 2K+(aq) + S2- (aq) → CuS(s) + 2K+(aq) + 2Cl-(aq)?
(Multiple Choice)
4.7/5  (36)
(36)
O2 can be produced by the reaction 2KClO3(s) → KCl(s) + 3O2(g). In one experiment, 0.312 g of O2 was formed. How much KClO3 was decomposed?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following definitions of reduction is generally most useful when dealing with ionic materials?
(Multiple Choice)
4.7/5  (38)
(38)
You have a sample of 3.01 × 1023 atoms of silver. How much does this sample weigh?
(Multiple Choice)
4.7/5  (36)
(36)
Ethanol is produced industrially by the acid-catalyzed reaction of ethylene with water. The balanced equation for this reaction is as follows: C2H4(g) + H2O(g) → C2H5OH(l)
If water is present in excess, how many moles of ethanol can be produced from 7.24 mol of ethylene?
(Multiple Choice)
4.9/5  (30)
(30)
Consider the following representation of a voltaic cell. 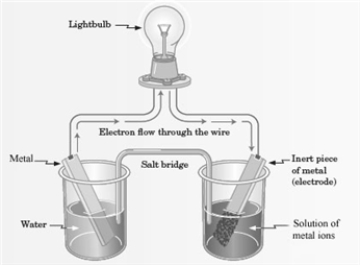 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the identity of the ion in the solution shown in the diagram?
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the identity of the ion in the solution shown in the diagram?
(Multiple Choice)
5.0/5  (38)
(38)
A black precipitate of copper sulfide, CuS, is formed when a solution of Cu(II) chloride, CuCl2, is added to a solution of potassium sulfide, K2S. Which of the following ionic equations for this reaction includes the spectator ions as well?
(Multiple Choice)
4.8/5  (39)
(39)
The metabolism of one mole of glucose, C6H12O6, releases 670 kcal of heat. How much heat is released by the combustion of 0.300 moles of glucose?
(Multiple Choice)
4.9/5  (29)
(29)
When solutions of AgNO3 and NaCl react, the balanced molecular equation is as follows: AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
How much AgCl is produced when 3.10 g of AgNO3 and 0.600 g of NaCl react?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 138
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)