Exam 2: An Introduction to the Chemical Basis of Life
Exam 1: Microbial World61 Questions
Exam 2: An Introduction to the Chemical Basis of Life37 Questions
Exam 3: The Biochemistry of Macromolecules59 Questions
Exam 4: Microscopy53 Questions
Exam 5: Prokaryote Organisms92 Questions
Exam 6: Eukaryotic Cells50 Questions
Exam 7: Eukaryotic Organisms50 Questions
Exam 8: Viruses and Infectious Particles72 Questions
Exam 9: Metabolism60 Questions
Exam 10: Microbial Genetics and Genetic Engineering99 Questions
Exam 11: Microbial Growth and Control108 Questions
Exam 12: Antimicrobial Agents145 Questions
Exam 13: Innate Immunity63 Questions
Exam 14: Adaptive Immunity70 Questions
Exam 15: Vaccination, Immunoassays, and Immune Disorders74 Questions
Exam 16: Microbial Pathogenesis95 Questions
Exam 17: Epidemiology and Infection Control74 Questions
Exam 18: Diseases of the Respiratory System50 Questions
Exam 19: Diseases of the Skin and Eyes49 Questions
Exam 20: Diseases of the Gastrointestinal System50 Questions
Exam 21: Diseases of the Urogenital System50 Questions
Exam 22: Diseases of the Nervous System49 Questions
Exam 23: Diseases of the Cardiovascular and Lymphatic Systems48 Questions
Exam 24: Environmental and Industrial Microbiology88 Questions
Select questions type
When two adjacent atoms of similar electronegativity share a pair of electrons, ________. (Select all that apply)
(Multiple Choice)
4.8/5  (32)
(32)
Acid rain is a serious environmental problem. A sample of rainwater collected in the Adirondack Mountains had an H+ concentration of 10-4 mol/L. The pH of this sample was ________.
(Multiple Choice)
4.8/5  (34)
(34)
When two adjacent atoms unequally share a pair of electrons, ________. (Select all that apply)
(Multiple Choice)
4.9/5  (36)
(36)
When a pair of valence shell electrons are shared by two atoms with significantly different electronegativity values, ________.
(Multiple Choice)
4.9/5  (41)
(41)
Glucose and fructose represent ________ isomers. (Select all that apply)
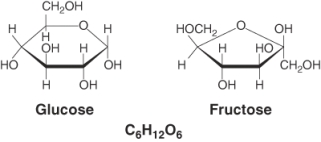
(Multiple Choice)
4.9/5  (32)
(32)
What is the chemical mechanism by which cells degrade polymers into monomers?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following represent ways to increase the molecular diversity of organic molecules? (Select all that apply)
(Multiple Choice)
4.7/5  (40)
(40)
The molecular weight of glucose is 181.18 g/mol. To make 250 ml of a 0.25 M glucose solution, you would dissolve ________ g of glucose in 250 ml of water.
(Multiple Choice)
4.8/5  (41)
(41)
When an atom loses a valence shell electron, it ________. (Select all that apply)
(Multiple Choice)
4.8/5  (47)
(47)
Hydrogen bonds between water molecules in solid form ________. (Select all that apply)
(Multiple Choice)
4.8/5  (36)
(36)
Each proton and neutron have a mass of one atomic mass unit (amu). Using this information from the periodic table, determine the mass of sodium (Na).

(Multiple Choice)
4.9/5  (47)
(47)
Which functional group acts as an acid by donating a hydrogen ion to the solution?
(Multiple Choice)
4.7/5  (39)
(39)
Showing 21 - 37 of 37
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)