Exam 6: Solids
Exam 1: Fundamentals57 Questions
Exam 2: The Language of Organic Chemistry25 Questions
Exam 3: Atomic Structure and Properties34 Questions
Exam 4: Diatomic Molecules27 Questions
Exam 5: Polyatomic Molecules30 Questions
Exam 6: Solids31 Questions
Exam 7: Acids and Bases27 Questions
Exam 8: Gases38 Questions
Exam 9: Reaction Kinetics50 Questions
Exam 10: Molecular Spectroscopy25 Questions
Exam 11: Analytical Chemistry24 Questions
Exam 12: Molecular Characterisation30 Questions
Exam 13: Energy and Thermochemistry47 Questions
Exam 14: Entropy and Gibbs Energy40 Questions
Exam 16: Electrochemistry24 Questions
Exam 17: Phase Equilibrium and Solutions25 Questions
Exam 18: Isomerism and Stereochemistry25 Questions
Exam 19: Organic Reaction Mechanisms28 Questions
Exam 20: Halogenoalkanes: Substitution and Elimination Reactions31 Questions
Exam 21: Alkenes and Alkynes: Electrophilic Addition and Pericyclic Reactions27 Questions
Exam 22: Benzene and Other Aromatic Compounds: Electrophilic Substitution Reactions37 Questions
Exam 23: Aldehydes and Ketones: Nucleophilic Addition and Α-Substitution Reactions33 Questions
Exam 24: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution and Α-Substitution Reactions25 Questions
Exam 25: Hydrogen30 Questions
Exam 26: S-Block Chemistry28 Questions
Exam 27: P-Block Chemistry33 Questions
Exam 28: D-Block Chemistry34 Questions
Select questions type
Use the Born Lande equation to calculate the lattice energy of MgO given that r = 210.5 pm.
(Multiple Choice)
4.8/5  (39)
(39)
Use the Kapustinskii equation to estimate the lattice enthalpy for KBr given that the ionic radii are 138 and 196 pm for K+ and Br- respectively.
(Multiple Choice)
4.8/5  (38)
(38)
The structure of the perovskite (calcium titanate) is given below. What is the coordination number of the two cations? Please select all that apply.
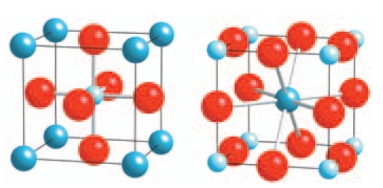
(Multiple Choice)
4.8/5  (32)
(32)
Match the value of Born exponent with the appropriate compound. given that the Born exponent values for the individual ions are Be2+ = 5, F- = 7, Zn2+, S2- and Cl- = 9, Rb+ = 10, Cs+ = 12 respectively.
-BeF2
(Multiple Choice)
4.8/5  (31)
(31)
Magnesium oxide crystallizes with the sodium chloride structure. Magnesium oxide obeys the radius ratio rule for this structure.
(True/False)
4.8/5  (42)
(42)
There is no structural analogue of CaF2 in hexagonal close packing (fill all tetrahedral holes).
(True/False)
4.9/5  (34)
(34)
Use the following data to construct a thermochemical cycle for the formation of ZnS from its elements and use the cycle to calculate the enthalpy of formation of ZnS.
Zn (s) Zn (g) 130 kJ mol-1
Zn (g) Zn+ (g) 906 kJ mol-1
Zn+ (g) Zn2+ (g) 1733 kJ mol-1
ZnS (s) Zn2+ (g) + S2- (g) 3048 kJ mol-1
S (s) S (g) 233 kJ mol-1
S (s) S- (g) -200 kJ mol-1
S- (s) S2- (g) 456 kJ mol-1
(Multiple Choice)
4.7/5  (33)
(33)
Match the value of Born exponent with the appropriate compound. given that the Born exponent values for the individual ions are Be2+ = 5, F- = 7, Zn2+, S2- and Cl- = 9, Rb+ = 10, Cs+ = 12 respectively.
-CsCl
(Multiple Choice)
4.9/5  (36)
(36)
Showing 21 - 31 of 31
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)