Exam 5: Polyatomic Molecules
Exam 1: Fundamentals57 Questions
Exam 2: The Language of Organic Chemistry25 Questions
Exam 3: Atomic Structure and Properties34 Questions
Exam 4: Diatomic Molecules27 Questions
Exam 5: Polyatomic Molecules30 Questions
Exam 6: Solids31 Questions
Exam 7: Acids and Bases27 Questions
Exam 8: Gases38 Questions
Exam 9: Reaction Kinetics50 Questions
Exam 10: Molecular Spectroscopy25 Questions
Exam 11: Analytical Chemistry24 Questions
Exam 12: Molecular Characterisation30 Questions
Exam 13: Energy and Thermochemistry47 Questions
Exam 14: Entropy and Gibbs Energy40 Questions
Exam 16: Electrochemistry24 Questions
Exam 17: Phase Equilibrium and Solutions25 Questions
Exam 18: Isomerism and Stereochemistry25 Questions
Exam 19: Organic Reaction Mechanisms28 Questions
Exam 20: Halogenoalkanes: Substitution and Elimination Reactions31 Questions
Exam 21: Alkenes and Alkynes: Electrophilic Addition and Pericyclic Reactions27 Questions
Exam 22: Benzene and Other Aromatic Compounds: Electrophilic Substitution Reactions37 Questions
Exam 23: Aldehydes and Ketones: Nucleophilic Addition and Α-Substitution Reactions33 Questions
Exam 24: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution and Α-Substitution Reactions25 Questions
Exam 25: Hydrogen30 Questions
Exam 26: S-Block Chemistry28 Questions
Exam 27: P-Block Chemistry33 Questions
Exam 28: D-Block Chemistry34 Questions
Select questions type
BeMe2 rapidly polymerizes to form a linear chain of tetrahedral Be atoms. The same process does not occur for BeBut2 due to steric hindrance and the shape at Be is linear. What sort of hybridization is present in each case?
(Multiple Choice)
4.8/5  (38)
(38)
In the BeH2 molecular orbital diagram shown below, the Be is double bonded to the H atoms.
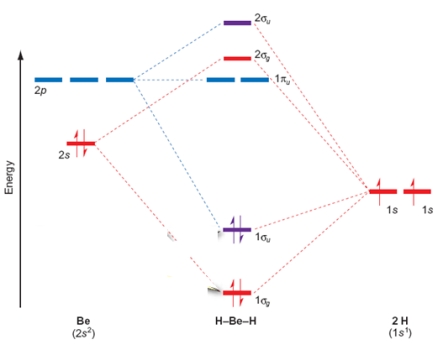
(True/False)
4.8/5  (33)
(33)
For each of the atoms highlighted in bold, determine the type of hybridization present.
-CH3CH2CCH
(Multiple Choice)
4.8/5  (38)
(38)
In the second period from Li to Ne, while no molecules exist which have more than eight electrons, there are some which exist with less. These molecules are described as electron ____
(Short Answer)
4.9/5  (42)
(42)
The bonding in B2H6 is best described by which of the following statements?
(Multiple Choice)
4.9/5  (33)
(33)
Use VSEPR theory to match the molecule with its shape.
-H2S
(Multiple Choice)
4.8/5  (31)
(31)
In VSEPR theory, the most important interactions that should be considered when placing bonding and lone pairs of electrons in a trigonal bipyramid occur at 90°.
(True/False)
4.8/5  (35)
(35)
A stream of trichloromethane (chloroform) will be deflected by a positive charge because the more electronegative ____ atoms generate dipoles which do not cancel each other out.
(Short Answer)
4.9/5  (42)
(42)
When predicting the shape of a molecule containing lone pairs, which of the following should be arranged first?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 21 - 30 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)