Exam 4: An Introduction to Organic Compounds
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
Which pairs of molecules can form a hydrogen bond with one another?
(Multiple Choice)
4.9/5  (21)
(21)
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-PO43-
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
(Essay)
4.8/5  (39)
(39)
Serotonin is a molecule involved in the transmission of nerve impulses. One of the organic families present in serotonin is ___. 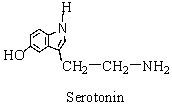
(Multiple Choice)
4.8/5  (33)
(33)
The shape of formaldehyde CH2O (C is the central atom), a substance often used in building insulation materials, is ___.
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following is the correct line-bond structure for the BrO3- ion?
(Multiple Choice)
4.9/5  (41)
(41)
The strongest noncovalent interaction that can occur between two methyl alcohol molecules is ___. 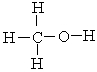
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following represents the correct line-bond structure of nitrosyl chloride (ONCl)? In this molecule, nitrogen is the central atom.
(Multiple Choice)
4.9/5  (34)
(34)
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-AsCl3
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
(Essay)
4.9/5  (32)
(32)
This molecule is responsible for the aroma of grape fruit. Which organic families are present?
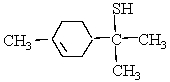
(Short Answer)
4.8/5  (28)
(28)
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-NO2-
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
(Essay)
4.8/5  (35)
(35)
Showing 21 - 40 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)