Exam 6: Oxidation-Reduction Reactions
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
The reaction, HCl(aq)+ Na2CO3(aq) NaCl(aq)+ H2O(l)+ CO2(g), involves changes in oxidation number and is therefore classified as a redox reaction.
(True/False)
4.8/5  (38)
(38)
Which substance in the reaction BrO3-(aq)+ 3 Zn(s)+ 6 H+(aq)→ Br-(aq)+ 3 Zn2+(aq)+ 3 H2O(l)is the oxidizing agent?
(Short Answer)
4.8/5  (34)
(34)
A student working on her research project will do a synthesis which involves bubbling chlorine gas through the aqueous reaction mixture in a closed system. The outlet from the system goes through a scrubber, which contains some sodium thiosulfate solution. The sodium thiosulfate will react with the chlorine passing through the scrubber to produce harmless chloride ion and sulfate ion. This prevents the chlorine from going up the exhaust system and polluting the atmosphere on campus grounds. The reaction is: Cl2(g)+ S2O32-(aq)→ SO42-(aq)+ Cl-(aq)The reaction calls for 15.5 grams of chlorine gas. The student wants enough thiosulfate in the scrubber to be able to handle twice this amount to be on the safe side. How many grams of crystal sodium thiosulfate (which is actually sodium thiosulfate pentahydrate)should the student weigh out for use in the scrubber? Hint: Make sure you balance the equation and focus on units.
(Short Answer)
4.8/5  (30)
(30)
Is the following, NO3 → NO, an oxidation or a reduction half-reaction?
(Short Answer)
4.9/5  (25)
(25)
When the carbohydrate, C12H22O11, undergoes complete combustion, the reducing agent in the reaction is
(Multiple Choice)
4.9/5  (35)
(35)
A partial activity series is: Au < Ag < Cu < H2 < Sn < Cd < Fe < Mn. Which metal(s)is/are NOT likely to be produced when each of the following compounds is made to react with Cd metal:Cd(NO3)2(aq); Fe(NO3)2(aq); HNO3(aq); Cu(NO3)2(aq); Sn(NO3)2(aq); AgNO3(aq)? Hint: In the partial activity series above, Mn is the most reactive and Au is the least reactive.
(Multiple Choice)
4.9/5  (44)
(44)
In the equation, CrO42-(aq) + H2O(l) CrO2-(aq)+ OH-(aq), the change in the oxidation number of the chromium atom is
(Multiple Choice)
4.9/5  (40)
(40)
Which statement is true concerning an oxidation-reduction reaction?
(Multiple Choice)
4.9/5  (30)
(30)
After balancing the following equation for the reaction in basic media,
H2O + CrO42-(aq)+ Br-(aq) CrO2-(aq)+ BrO3-(aq)+ OH- what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
(Multiple Choice)
4.8/5  (41)
(41)
Fe2+(aq)reacts with MnO4-(aq)ion in acidic solution to yield Fe3+(aq)ions and Mn2+(aq)ions. 5Fe2+(aq)+ MnO4-(aq)+ 8H+ 5Fe3+(aq)+ Mn2+(aq)+ 4H2O Melanterite is a greenish mineral, and can be found on the walls of mines. A sample of melanterite, FeSO4·7H2O, was analyzed for purity using this reaction by titration of an aqueous solution of the sample. One such sample required 56.15 mL of 0.01301 M permanganate solution to completely titrate all the iron in the sample by the reaction shown above. How much did the sample weigh, if it was in fact pure melanterite? Hint: Watch your units carefully at each step of the problem.
(Multiple Choice)
4.9/5  (35)
(35)
Balance the redox reaction below in acidic solution.PbO2(s)+ I-(aq)→ Pb2+(aq)+ I2(s)When balanced, what is the sum of all the coefficients? Hint: Do not neglect to include electrons when balancing redox reactions.
(Short Answer)
4.8/5  (38)
(38)
A solution was made by taking 2.500 g of KMnO4 and dissolving it in enough water to make 1.000 liter of solution. This solution was used to titrate H2C2O4·2H2O, a very pure substance. In acidic media, the reaction is 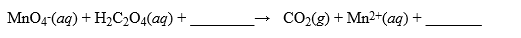 How many mL of this solution are required to titrate a 0.480 g sample of H2C2O4·2H2O? Hint: Remember to use moles as an intermediary when performing your stoichiometry calculations for the titration.
How many mL of this solution are required to titrate a 0.480 g sample of H2C2O4·2H2O? Hint: Remember to use moles as an intermediary when performing your stoichiometry calculations for the titration.
(Short Answer)
4.8/5  (38)
(38)
When hydrocarbons or coal contain sulfur what is formed during the complete combustion? Hint: Consider the products of a complete combustion reaction with something containing sulfur. What should always be produced?
(Multiple Choice)
4.8/5  (33)
(33)
A partial activity series is: Pb < Sn < Co < Fe < Zn. Which metal will react least readily with nonoxidizing acids to produce hydrogen gas? Hint: Oxidation is a loss of electrons.
(Short Answer)
4.8/5  (38)
(38)
What is the change in the oxidation number of manganese in the following? KMnO4 MnSO4
(Multiple Choice)
4.8/5  (39)
(39)
Complete the balancing of the following half-reaction, taking place in basic media. How many electrons are needed to balance the half-reaction?
Cr(OH)4(aq) CrO42(aq)Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
(Multiple Choice)
4.8/5  (35)
(35)
In the equation, 2H+ (aq)+ Zn(s) H2(g)+ Zn2+(aq), the H+ is the oxidizing agent and Zn is the reducing agent.
(True/False)
4.9/5  (32)
(32)
SO2 is a dangerous air pollutant, which can harm human lungs. The quantity of SO2 in air can be determined by the reaction,
2 MnO4-(aq)+ 5 SO2(g)+ 2 H2O 5 SO42-(aq)+ 2 Mn2+(aq)+ 4 H+ It required 185 mL of 0.0200 M MnO4-(aq)solution to completely react with all of the SO2 in a sample of air. How many grams of SO2 were in the sample?
(Multiple Choice)
4.8/5  (31)
(31)
Showing 61 - 80 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)