Exam 6: Oxidation-Reduction Reactions
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
In order for an oxidation reaction to occur, a reduction reaction must also take place.
(True/False)
4.8/5  (37)
(37)
The nitrogen molecule, (N2), was involved in a chemical reaction in which it underwent reduction. Based on the change in oxidation numbers, which of the species listed below is a possible product of the reaction?
(Multiple Choice)
4.8/5  (38)
(38)
The following reaction describes the decomposition of NaHCO3, baking soda:2NaHCO3 Na2CO3 + CO2 + H2O This is not an oxidation-reduction reaction, since nothing is oxidized or reduced.
(True/False)
4.7/5  (39)
(39)
When H2O2 behaves as a reducing agent, the most likely product formed from it is ________. Hint: Write out an example equation with H2O2 being oxidized to see the result.
(Short Answer)
4.8/5  (30)
(30)
The reaction, AgNO3(aq)+ NH4Br(aq) AgBr(s)+ NH4NO3(aq), involves changes in oxidation number and is therefore classified as a redox reaction.
(True/False)
4.9/5  (42)
(42)
Complete the balancing of the following half-reaction, taking place in basic media. How many hydroxide ions are needed to balance the half-reaction?Br(aq)s BrO3(aq)Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
(Multiple Choice)
4.7/5  (33)
(33)
When the equation, Al(s)+ NO3-(aq)→ NO(g)+ Al3+(aq)is balanced for an acidic solution, the sum of ALL the coefficients is ________. Hint: Do not neglect to include electrons when balancing redox reactions.
(Short Answer)
4.8/5  (37)
(37)
A partial activity series is: Au < Pt < Ag < Cu < H2 < Sn < Co < Fe < Mg. Based on this series, which of the following solutions would cause a reaction with Cu(s)? 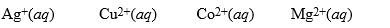
(Short Answer)
4.8/5  (44)
(44)
When a copper metal reacts with aqueous nitric acid, which substance is the oxidizing agent?
(Short Answer)
4.8/5  (35)
(35)
The CrO42- ion was involved in a chemical reaction, during which it is transformed into Cr3+ ions. What is the change in oxidation number of the chromium atom?
(Multiple Choice)
4.8/5  (37)
(37)
Balance the half-reaction, C2H6O HC2H3O2, taking place in acidic media. How many electrons are needed to balance the charge?Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
(Multiple Choice)
4.9/5  (40)
(40)
The ClO2 molecule was involved in a chemical reaction in which it underwent oxidation. Based on the change in oxidation numbers, which of the species listed below is a possible product of the reaction?
(Multiple Choice)
4.8/5  (32)
(32)
Balance the redox reaction below in acidic solution.Cl2(g)→ Cl-(aq)+ ClO-(aq)When balanced, what is the sum of all the coefficients? Hint: Consider your oxidation numbers when balancing redox reactions.
(Short Answer)
4.7/5  (42)
(42)
Balance the redox reaction below in basic solution.PbO2(s)+ I-(aq)→ Pb2+(aq)+ I2(s)What is the coefficient of OH- in the final balanced equation? Hint: Consider your oxidation numbers when balancing redox reactions.
(Short Answer)
5.0/5  (39)
(39)
The following chemical equation shows how water can be produced.
H2(g)+ ½ O2(g) H2O(l)In the reaction, hydrogen is the species which is reduced.
(True/False)
4.7/5  (27)
(27)
Zinc metal reacts with perchloric acid solution to produce aqueous zinc perchlorate and hydrogen gas, which escapes. The species being oxidized in this reaction is
(Multiple Choice)
4.8/5  (30)
(30)
Showing 21 - 40 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)