Exam 5: Ionic and Metallic Bonds
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
What is the formula of the neutral compound which might be formed from P5+ and S2-?
(Multiple Choice)
4.8/5  (36)
(36)
The bond-type triangle can be used for
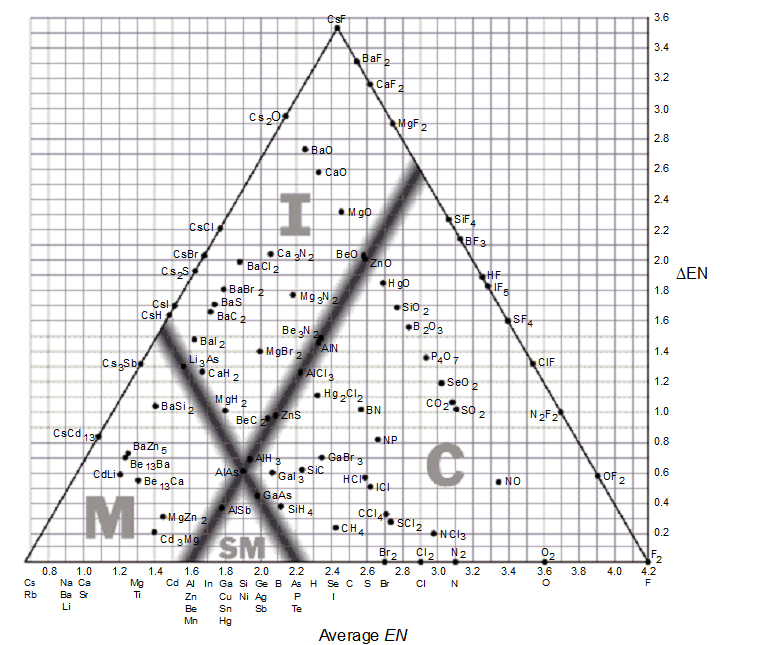 -Based on the bond-type triangle, which of these covalent compounds has the greatest ionic character?
-Based on the bond-type triangle, which of these covalent compounds has the greatest ionic character?
(Multiple Choice)
4.8/5  (42)
(42)
What would be the product of the reaction between aluminum metal and sulfur?
(Multiple Choice)
4.8/5  (24)
(24)
In the following oxidation-reduction reaction, which of the following statements is true?
Sr(s) + 2 H2O(l) Sr2+(aq) + 2 OH-(aq) + H2(g)
(Multiple Choice)
4.9/5  (39)
(39)
Honda and Toyota both sell hybrid cars that have engines that can run on either gasoline or electricity stored in a battery. The advantage of hybrid cars is simple: Totally electric cars need batteries that weigh almost as much as the vehicle! It isn't surprising that a high priority is the search for better batteries. Barium ferrate (BaFeO4) is being tested for use in batteries. Use the positions of barium and oxygen in the periodic table to predict the oxidation state of the iron atom in this compound.
(Multiple Choice)
4.9/5  (36)
(36)
If the formula for aluminum oxide is Al2O3, what would be the formulas for
aluminum fluoride and aluminum sulfide?
(Short Answer)
4.9/5  (42)
(42)
Which of the following metals is the most "active," the metal which should react most rapidly with oxygen or water vapor in the atmosphere?
(Multiple Choice)
5.0/5  (25)
(25)
The bond-type triangle can be used for
 -As you move from the bottom to the top on a bond-type triangle, _________.
-As you move from the bottom to the top on a bond-type triangle, _________.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following sets of elements is arranged in order of increasing
Metallic character?
(Multiple Choice)
4.7/5  (43)
(43)
Aluminum chlorhydrate is added to antiperspirants to stop people from sweating. If this compound contains neutral H2O molecules and Al3+, OH-, and Cl- ions, and the formula of this compound is Alx(OH)5Cl • 2 H2O, what is the value of x in this formula?
(Multiple Choice)
4.7/5  (37)
(37)
An element X forms a compound with oxygen, X2O3, and with hydrogen, XH3. The element X must be in which group in the periodic table?
(Multiple Choice)
4.9/5  (34)
(34)
An element, X, forms the ionic compound CaX. X is a member of:
(Multiple Choice)
4.7/5  (43)
(43)
Predict the product of the following reaction: Sr(s) + P4(s).
(Multiple Choice)
4.9/5  (34)
(34)
Showing 41 - 60 of 80
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)