Exam 5: Ionic and Metallic Bonds
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Which of the following elements would be the most likely to form an oxide with the formula XO and a hydride with the formula XH2 (X represents the unknown element)?
(Multiple Choice)
4.8/5  (33)
(33)
Lithium ion batteries are high energy density 3.6 V batteries that use a
Lithium salt such as LiCoO2 or LiPF6 for the positive electrode, or cathode. Use the positions of lithium and fluorine in the periodic table to predict the oxidation state of the phosphorus atom in LiPF6.
(Multiple Choice)
4.7/5  (40)
(40)
Use the positions of silicon and fluorine in the periodic table to predict the most likely product of the reaction between silicon and fluorine.
(Multiple Choice)
4.7/5  (39)
(39)
The bond-type triangle can be used for
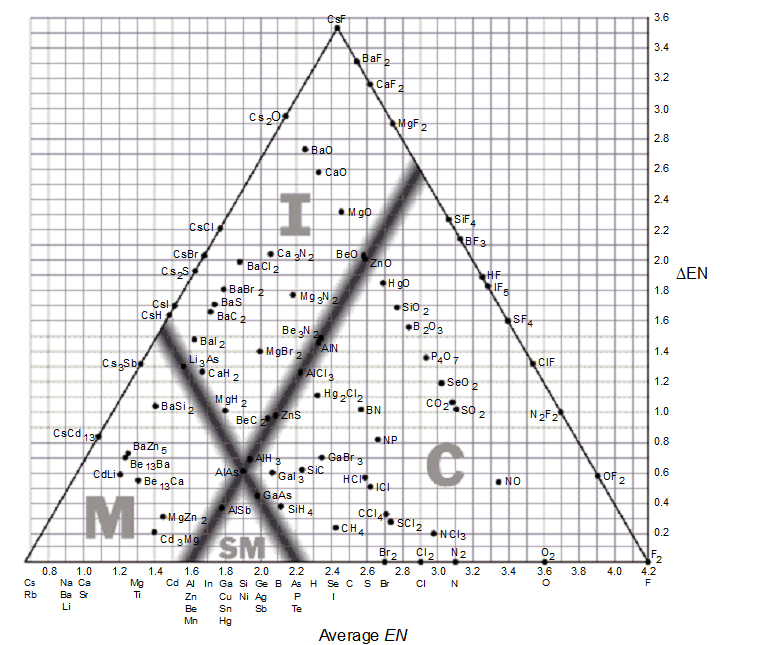 -Describe what happens to the distribution of electrons in the bond between two elements as you move from left to right on a bond-type triangle. Describe what happens to the distribution of the electrons in a bond between two elements as you move from the bottom to the top of a bond-type triangle.
-Describe what happens to the distribution of electrons in the bond between two elements as you move from left to right on a bond-type triangle. Describe what happens to the distribution of the electrons in a bond between two elements as you move from the bottom to the top of a bond-type triangle.
(Essay)
4.8/5  (38)
(38)
Which element is hydrogen most like to combine with to form a hydride?
(Multiple Choice)
4.8/5  (34)
(34)
What is the most likely electronic configuration of the Co2+ ion?
(Multiple Choice)
4.7/5  (31)
(31)
Determine the oxidation number of sulfur in sulfurous acid, H2SO3.
(Multiple Choice)
4.9/5  (39)
(39)
Identify the reducing agent and oxidizing agent in the reaction:
H2O2(aq) + 2 HI(aq) 2 H2O(l) + I2(s)
(Essay)
4.9/5  (46)
(46)
A mineral called montmorillonite will catalyze some interesting reactions. The formula of one type of montmorillonite is NaCa4(Si2O5)4F. If Na has a charge of +1, Ca has a charge of +2, and F has a charge of -1, what is the charge on each Si2O5 complex ion?
(Multiple Choice)
4.8/5  (36)
(36)
The bond-type triangle can be used for
 -Use a bond-type triangle to predict the correct arrangement of the following compounds in order of increasing covalent character.
Mg Zn2, CCl4, SiH4
-Use a bond-type triangle to predict the correct arrangement of the following compounds in order of increasing covalent character.
Mg Zn2, CCl4, SiH4
(Multiple Choice)
4.9/5  (31)
(31)
Determine the oxidation number of the central carbon atom in the following compound.
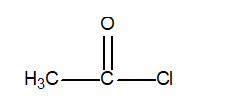
(Multiple Choice)
4.9/5  (37)
(37)
The bond-type triangle can be used for
 -Using a bond-type triangle, predict which of the following compounds would have approximately 50% ionic bonding and 50% covalent bonding.
-Using a bond-type triangle, predict which of the following compounds would have approximately 50% ionic bonding and 50% covalent bonding.
(Multiple Choice)
5.0/5  (29)
(29)
In the following redox reaction which element is being oxidized?
Cu(s) + 4 HNO3(aq) Cu(NO3)2 (aq) + 2 NO2
(Multiple Choice)
4.7/5  (48)
(48)
Predict the formulas for neutral compounds containing the following pairs of ions.
A) H+ and O22-
B) Zn2+ and PO43-
C) K+ and [PtCl6]2-
(Short Answer)
4.8/5  (40)
(40)
What group of metals react with sulfur to form M2S3 sulfides, reacts with fluorine to form MF3 fluorides, and react with acid to form M3+ ions and H2 gas?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following metals would be the most reactive towards air and water?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following is the most likely product of the reaction between magnesium metal and nitrogen?
(Multiple Choice)
5.0/5  (53)
(53)
The two most common ions of copper are Cu1+ and Cu2+. Give the most likely electron configuration of each of these ions.
(Short Answer)
4.8/5  (30)
(30)
The bond-type triangle can be used for
 -Use a bond-type triangle to describe the bonding in CaH2.
-Use a bond-type triangle to describe the bonding in CaH2.
(Multiple Choice)
4.7/5  (33)
(33)
Showing 21 - 40 of 80
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)