Exam 5: Ionic and Metallic Bonds
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Which of the following formula/name combinations is incorrect?
(Multiple Choice)
4.8/5  (42)
(42)
Predict the formula of the electrically neutral compound formed from the
Following ions: Ca2+ and PO43-.
(Multiple Choice)
4.9/5  (38)
(38)
Predict the formula of the neutral ionic compound formed from K+ and S2-.
(Multiple Choice)
4.8/5  (34)
(34)
Predict the product of the reaction between barium and nitrogen.
(Multiple Choice)
4.8/5  (27)
(27)
In Chapter 4 we were able to determine the geometric shape and resulting polarity of individual covalent molecules. In Chapter 5 we did not do this for ionic and metallic compounds. Why not?
(Multiple Choice)
4.9/5  (46)
(46)
Use the positions of gallium and oxygen in the periodic table to predict the
Formula for gallium oxide.
(Multiple Choice)
4.7/5  (30)
(30)
The bond-type triangle can be used for
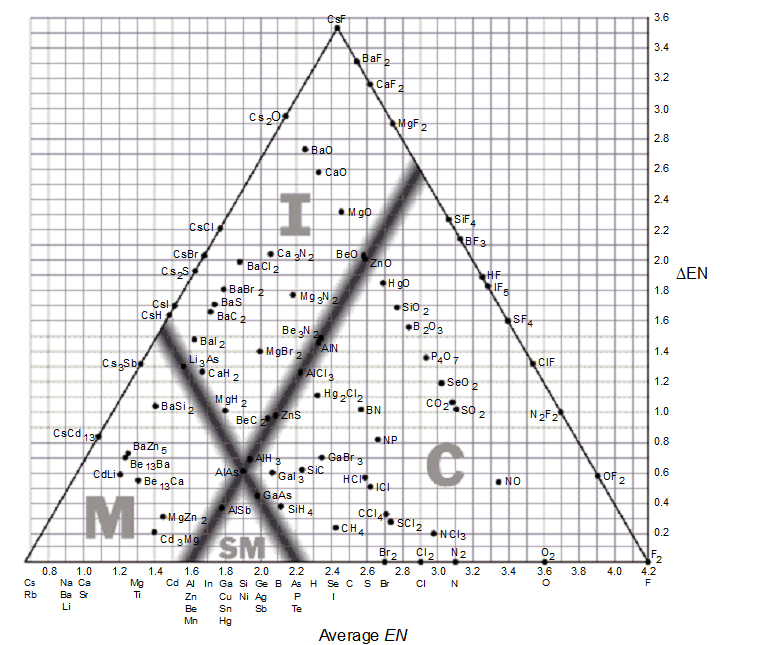 -According to the supplied bond-type triangle, which of the following compounds is most likely to conduct electricity as a solid?
-According to the supplied bond-type triangle, which of the following compounds is most likely to conduct electricity as a solid?
(Multiple Choice)
4.8/5  (42)
(42)
If the difference in electronegativity between two elements in a compound is very small (less then 0.2), can you safely conclude that the compound formed from the two elements is covalent?
(Multiple Choice)
4.8/5  (36)
(36)
A main-group reacts with hydrogen and oxygen to form compounds with the formulas XH4 and XO2 (X represents the metal). In which group of the periodic table does this element belong?
(Multiple Choice)
4.8/5  (40)
(40)
All but one of the following species contains nitrogen in the same oxidation state. Which one is different?
(Multiple Choice)
4.9/5  (37)
(37)
The bond-type triangle can be used for
 -Based on the given bond-type triangle, what is the highest possible average electronegativity a compound can have and still retain some metallic character?
-Based on the given bond-type triangle, what is the highest possible average electronegativity a compound can have and still retain some metallic character?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 61 - 80 of 80
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)