Exam 5: Periodicity and the Atomic Structure of Atoms
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Compared to sulfur,chlorine has a ________ effective nuclear charge,Zeff,and a ________ atomic radius.
(Short Answer)
4.9/5  (31)
(31)
What is the de Broglie wavelength of a 300-g object moving at a velocity of 50 m/s (about 100 mph)?
(Multiple Choice)
4.9/5  (34)
(34)
According to the Balmer-Rydberg equation,electromagnetic radiation with the shortest wavelength will be emitted when an electron undergoes which of the following transitions?
(Multiple Choice)
4.9/5  (39)
(39)
A person is most likely to experience serious biological effects when exposed to which of the following forms of electromagnetic radiation?
(Multiple Choice)
4.8/5  (36)
(36)
Which orbitals have two nodal planes passing through the nucleus?
(Multiple Choice)
4.8/5  (41)
(41)
Of the following,which atom has the smallest atomic radius?
(Multiple Choice)
4.9/5  (47)
(47)
Which of the following represent electron configurations that violate the Pauli exclusion principle?
(A)[Ne]3s13p5
(B)[Kr]4d125s25p3
(C)[Ar]3d104s24p2
(Multiple Choice)
4.9/5  (47)
(47)
Light can be made to have a higher intensity by raising its
(Multiple Choice)
4.9/5  (38)
(38)
Which element has the ground-state electron configuration [Xe]6s25d14f7?
(Multiple Choice)
4.7/5  (34)
(34)
The spheres below represent atoms of Li,Be,B,and F (not necessarily in that order). 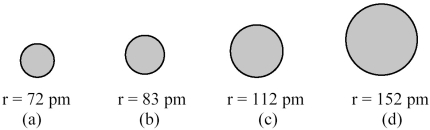 -Which one of these spheres represents an atom of F?
-Which one of these spheres represents an atom of F?
(Multiple Choice)
4.9/5  (41)
(41)
Which orbital-filling diagram represents the ground state of vanadium?
(Multiple Choice)
4.9/5  (33)
(33)
The Balmer-Rydberg equation can be extended to ions with only one electron,such as He+.In that case it has the form: 1/λ = Z2R(1/m2 - 1/n2),where Z is the atomic number.What is the energy of the photon required to promote an electron in He+ from a 1s orbital to a 2p orbital?
(Multiple Choice)
4.9/5  (37)
(37)
Which atom in each group (I and II)has the smallest atomic radius?
(I)Sr,Zr,I (II)N,P,As
(Multiple Choice)
4.9/5  (37)
(37)
What are the possible values of n and ml for an electron in a 5d orbital?
(Multiple Choice)
4.9/5  (34)
(34)
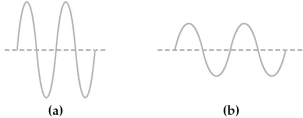 -If wave (a)represents green light,wave (b)might represent
-If wave (a)represents green light,wave (b)might represent
(Multiple Choice)
4.8/5  (38)
(38)
Middle C on a piano has a frequency of 262 Hz.In a vacuum 262 Hz has a corresponding wavelength of ________ m.
(Short Answer)
4.8/5  (42)
(42)
For hydrogen,what is the wavelength of the photon emitted when an electron drops from a 4d orbital to a 2p orbital in a hydrogen atom? The Rydberg constant is 1.097 × 10-2 nm-1.
(Multiple Choice)
4.8/5  (40)
(40)
The property of a wave that is associated with brightness or intensity is ________.
(Short Answer)
4.9/5  (42)
(42)
Showing 101 - 120 of 158
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)