Exam 5: Periodicity and the Atomic Structure of Atoms
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
The absorption of light of frequency 1.16 × 1011 Hz is required for CO molecules to go from the lowest rotational energy level to the next highest rotational energy level.Determine the energy for this transition in kJ/mol.h = 6.626 × 10-34 J ∙ s
(Multiple Choice)
4.8/5  (30)
(30)
Atoms of which element,indicated by letter on the periodic table,have the orbital-filling diagram shown below? 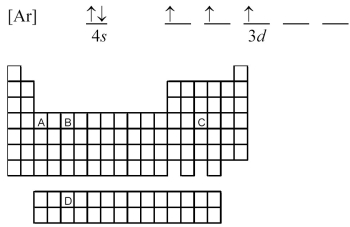
(Multiple Choice)
4.7/5  (31)
(31)
The average bond dissociation energy of a carbon-carbon bond is 410 kJ/mol.What wavelength in nanometers of ultraviolet radiation has an energy of 410 kJ/mol?
(Short Answer)
4.8/5  (28)
(28)
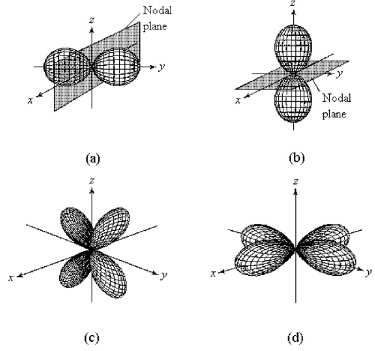 -Which of the above fourth-shell orbitals is a 4pz orbital?
-Which of the above fourth-shell orbitals is a 4pz orbital?
(Multiple Choice)
4.9/5  (39)
(39)
![-Which element,indicated by letter on the periodic table above,has the ground-state electron configuration [Ar]4s<sup>2</sup> 3d<sup>2</sup>?](https://storage.examlex.com/TB4939/11ea7a38_c8ee_2934_aa4c_2fa80ddc7ebb_TB4939_00_TB4939_00.jpg) -Which element,indicated by letter on the periodic table above,has the ground-state electron configuration [Ar]4s2 3d2?
-Which element,indicated by letter on the periodic table above,has the ground-state electron configuration [Ar]4s2 3d2?
(Multiple Choice)
4.9/5  (34)
(34)
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 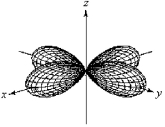
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following have their valence electrons in the same shell?
(Multiple Choice)
4.8/5  (30)
(30)
For a particular orbital,as one goes away from the nucleus along the z-axis,the probability density decreases to zero,then increases,and finally decreases without increasing a second time.This is consistent with a
(Multiple Choice)
4.9/5  (48)
(48)
The spheres below represent atoms of Sb,As,P,and N (not necessarily in that order). 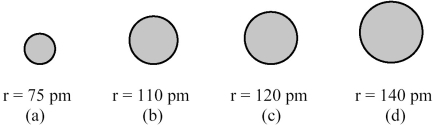 -Which one of these spheres represents an atom of Sb?
-Which one of these spheres represents an atom of Sb?
(Multiple Choice)
4.7/5  (39)
(39)
The spheres below represent atoms of Li,Be,B,and F (not necessarily in that order). 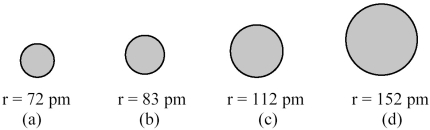 -Which one of these spheres represents an atom of Be?
-Which one of these spheres represents an atom of Be?
(Multiple Choice)
4.9/5  (41)
(41)
If the quantum number ms had possible values ±1,±2,what would be the maximum number of electrons that be placed in a single orbital?
(Multiple Choice)
4.8/5  (33)
(33)
 -Which of the above fourth-shell orbitals is a 4dx2-y2 orbital?
-Which of the above fourth-shell orbitals is a 4dx2-y2 orbital?
(Multiple Choice)
5.0/5  (30)
(30)
Showing 141 - 158 of 158
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)