Exam 5: Periodicity and the Atomic Structure of Atoms
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Photochemists use electromagnetic radiation to initiate chemical reactions,often by providing the energy required to break bonds within a molecule.Lowering which of the following will result in electromagnetic radiation having more energy per photon?
(Multiple Choice)
5.0/5  (41)
(41)
The subshell designations follow the alphabet after f.What is the first shell in which an h orbital would be allowed?
(Multiple Choice)
4.8/5  (32)
(32)
Which period of elements,indicated by letter on the periodic table,has electrons whose highest principal quantum number n is 5? 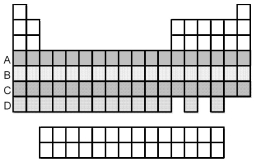
(Multiple Choice)
4.8/5  (35)
(35)
According to the Balmer-Rydberg equation,which transition results in the emission of a photon in the ultraviolet region of the electromagnetic radiation spectrum?
(Multiple Choice)
4.8/5  (39)
(39)
List all the elements that have a ground-state configuration with five unpaired electrons in the 3d subshell.
(Multiple Choice)
4.9/5  (30)
(30)
A radio station that broadcasts at 99.5 MHz is broadcasting at a frequency of ________ s-1.
(Short Answer)
4.8/5  (39)
(39)
Which of the following represent electron configurations that are allowed but do not represent ground-state configurations?
(A)[Ne]3s13p5 (B)[Kr]4d125s25p3 (C)[Ar]3d104s24p2
(Multiple Choice)
4.7/5  (35)
(35)
Which groups of elements,indicated by letter on the periodic table,have two unpaired p electrons in their valence shell? 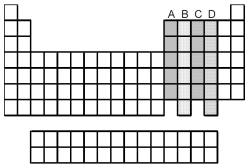
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following have the same number of valence electrons?
(Multiple Choice)
4.9/5  (47)
(47)
Atoms of which element,indicated by letter on the periodic table,have the orbital-filling diagram shown below? 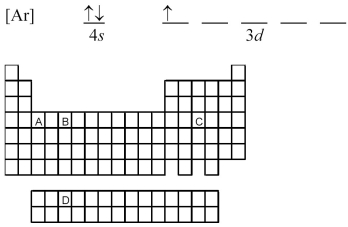
(Multiple Choice)
4.8/5  (44)
(44)
Which element has the ground-state electron configuration [ Rn] 7s26d2 ?
(Multiple Choice)
4.9/5  (38)
(38)
An electron in a 4p orbital can have a wave function with which of the following set of quantum numbers, (n,l,ml,ms)?
(Multiple Choice)
4.9/5  (33)
(33)
Compared to ultraviolet radiation,infrared radiation occurs at ________ wavelengths,________ frequencies,and ________ energies.
(Short Answer)
4.7/5  (29)
(29)
Which have the largest number of unpaired electrons in p orbitals in their ground-state electron configurations?
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following have their valence electrons in the same shell?
(Multiple Choice)
5.0/5  (38)
(38)
Showing 121 - 140 of 158
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)