Exam 7: Covalent Bonds and Molecular Structure
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
What are the bond angles in the following molecular model of TeF5-? 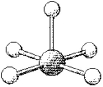
(Multiple Choice)
4.9/5  (35)
(35)
What is the geometry around the central atom in the following molecular model of SO32-? 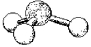
(Multiple Choice)
4.9/5  (41)
(41)
What are the bond angles in the following molecular model of SCl4? 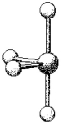
(Multiple Choice)
4.8/5  (41)
(41)
Shown below is a model of CH4 having an orientation in which one or more atoms are hidden from view.What is the indicated bond angle? 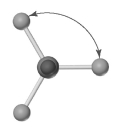
(Multiple Choice)
4.8/5  (38)
(38)
A reactive element with a relatively high electronegativity would be expected to have a relatively
(Multiple Choice)
5.0/5  (49)
(49)
What are the bond angles in the following molecular model of SO32-? 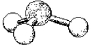
(Multiple Choice)
4.9/5  (33)
(33)
What are the bond angles in the following molecular model of XeF4? 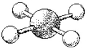
(Multiple Choice)
4.8/5  (36)
(36)
In the best Lewis structure for NO +,what is the formal charge on the N atom?
(Multiple Choice)
4.9/5  (32)
(32)
The electronegativity is 2.1 for H and 1.9 for Pb.Based on these electronegativities PbH4 would be expected to
(Multiple Choice)
4.9/5  (35)
(35)
Which molecule has a central atom that uses the set of hybrid orbitals shown below to form bonds with the non-central atoms? 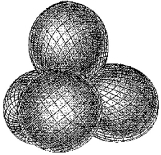
(Multiple Choice)
4.8/5  (34)
(34)
Assign formal charges to all atoms in the following resonance form for HNO3. 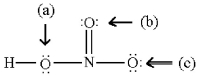
(Multiple Choice)
4.7/5  (21)
(21)
Showing 41 - 60 of 232
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)