Exam 7: Covalent Bonds and Molecular Structure
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
The electronegativities for the elements vary from 0.7 for cesium to 4.0 for fluorine.The electronegativity for iodine is 2.5.Based entirely on the general guidelines for electronegativities and bond character,
(Multiple Choice)
4.9/5  (40)
(40)
Which drawing represents a σ* antibonding molecular orbital for a homonuclear diatomic molecule?
(Multiple Choice)
4.7/5  (29)
(29)
Based on the indicated electronegativities,arrange the following in order of increasing ionic character: CsBr,LaBr3,PBr3,MgBr2. 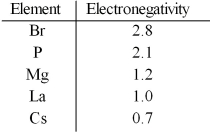
(Multiple Choice)
4.8/5  (41)
(41)
Of BrF3 and PF3,the one with the smaller bond angles is ________.
(Short Answer)
4.8/5  (29)
(29)
The following MO diagram is appropriate for Li2 and Be2.Based on this diagram, 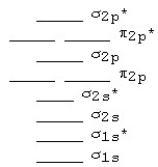
(Multiple Choice)
4.9/5  (36)
(36)
Of NH4+ and NH4- the one with the smaller bond angles is ________.
(Short Answer)
4.9/5  (35)
(35)
What geometric arrangement of charge clouds is expected for an atom that has four charge clouds?
(Multiple Choice)
4.9/5  (36)
(36)
What are the bond angles in the following molecular model of ICl4-? 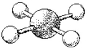
(Multiple Choice)
4.9/5  (41)
(41)
The electronegativity for both sulfur and carbon is 2.5.Therefore the compound CS2 would be expected to
(Multiple Choice)
4.8/5  (39)
(39)
Which drawing represents a π bonding molecular orbital for a homonuclear diatomic molecule?
(Multiple Choice)
4.7/5  (38)
(38)
Which molecule contains the most easily broken carbon-carbon bond?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following would be expected to have sp2 hybridization on atom A? 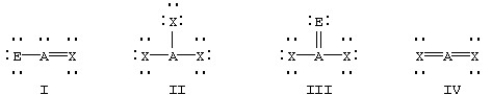
(Multiple Choice)
4.9/5  (30)
(30)
What are the bond angles in the following molecular model of BrF3? 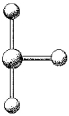
(Multiple Choice)
4.9/5  (41)
(41)
What is the geometry around the central atom in the following molecular model of ICl4-? 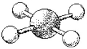
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following contains an atom that does not obey the octet rule?
(Multiple Choice)
4.8/5  (36)
(36)
What is the geometry around the central atom in the following molecular model of BCl3? 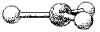
(Multiple Choice)
4.9/5  (33)
(33)
Showing 161 - 180 of 232
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)