Exam 7: Covalent Bonds and Molecular Structure
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Which is the most acceptable electron dot structure for N2H2?
(Multiple Choice)
5.0/5  (40)
(40)
Two resonance forms for SOCl2 are given below. 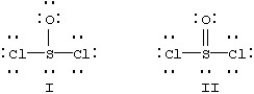 Which is favored by the octet rule and which by formal charge considerations?
Which is favored by the octet rule and which by formal charge considerations?
(Multiple Choice)
4.9/5  (36)
(36)
Which molecule has a central atom that uses the set of hybrid orbitals shown below to form bonds with the non-central atoms? 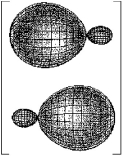
(Multiple Choice)
4.8/5  (34)
(34)
How many lone pairs of electrons are on the Xe atom in XeF6?
(Multiple Choice)
4.8/5  (42)
(42)
Use the following MO diagram for Be2,Be2+,and Be2-.Based on this diagram, 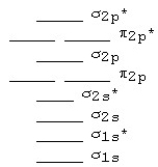
(Multiple Choice)
4.8/5  (43)
(43)
Predict whether removing two electrons from F2 will create an ion that is diamagnetic or paramagnetic and have an F-F that is stronger or weaker than the bond in F2.
(Short Answer)
4.8/5  (41)
(41)
Using only the elements Ba,F,and P,give the formula of a compound having largely polar covalent bonds.
(Short Answer)
5.0/5  (28)
(28)
The carbon-carbon bond in C2H2 contains ________ σ and ________ π bonds.
(Short Answer)
4.8/5  (42)
(42)
What is the geometry around the central atom in the following molecular model of H2S? 
(Multiple Choice)
4.8/5  (40)
(40)
The number of sp2 hybrid orbitals on the carbon atom in CO32- is
(Multiple Choice)
4.7/5  (36)
(36)
What are the bond angles in the following molecular model of PH3? 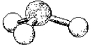
(Multiple Choice)
4.8/5  (42)
(42)
Electrostatic potential maps use color to portray the calculated electron distribution in a molecule.Atoms that are electron poor and carry a δ+ charge are shown in blue.Atoms that are electron rich and carry a δ- charge are shown in red.Atoms with little or no charge are shown in green.The electrostatic potential map of CH3Li below should show 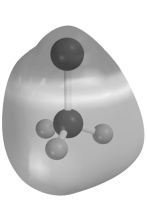
(Multiple Choice)
4.9/5  (36)
(36)
Showing 201 - 220 of 232
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)