Exam 5: Solution Concentration
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L. What is the molarity of the solution?
(Multiple Choice)
4.8/5  (37)
(37)
Consider the following structure. 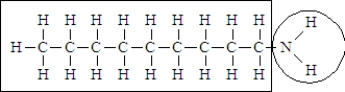 Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
-This molecule is probably____________________in water.
Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
-This molecule is probably____________________in water.
(Short Answer)
4.8/5  (32)
(32)
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 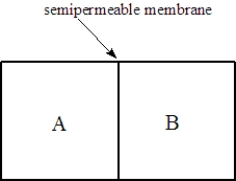 Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is pure water, and solution B is 0.05 M glucose. Water molecules will move to the ______________________compartment and glucose molecules will move to the __________________compartment.
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is pure water, and solution B is 0.05 M glucose. Water molecules will move to the ______________________compartment and glucose molecules will move to the __________________compartment.
(Short Answer)
4.9/5  (39)
(39)
The following list gives the concentration of the major components of blood plasma.
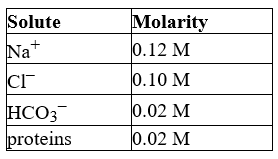 What is the total concentration of all the minor components in the plasma?
What is the total concentration of all the minor components in the plasma?
(Multiple Choice)
4.8/5  (47)
(47)
For the following questions,fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
-As the size of the hydrophobic portion of a molecule increases, the solubility in water will ___________.
(Short Answer)
4.8/5  (51)
(51)
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown. 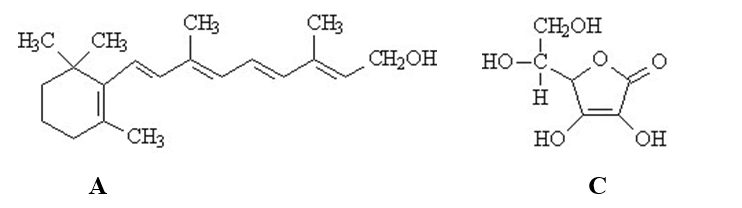 Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The beaker below contains oil (a compound of mostly carbon and hydrogen) in the upper layer and water in the lower layer. Oil floats on top of the water because it is less dense.
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The beaker below contains oil (a compound of mostly carbon and hydrogen) in the upper layer and water in the lower layer. Oil floats on top of the water because it is less dense.  The vitamin that would be the most soluble in the upper layer is ______.
The vitamin that would be the most soluble in the upper layer is ______.
(Short Answer)
4.8/5  (33)
(33)
How many grams of solid KCl are needed to prepare 250.0 mL of 0.235 M solution?
(Multiple Choice)
5.0/5  (42)
(42)
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.50 M in sucrose and Solution is 1.5 M in sucrose. After one hour, the compartment on the ____________________ will have the higher osmotic pressure.
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.50 M in sucrose and Solution is 1.5 M in sucrose. After one hour, the compartment on the ____________________ will have the higher osmotic pressure.
(Short Answer)
4.7/5  (36)
(36)
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
-Upon standing the glucose molecules will move in which direction?
Answer the following questions as appropriate with: right,left,or no movement.
-Upon standing the glucose molecules will move in which direction?
(Short Answer)
4.7/5  (28)
(28)
The solubility of a compound in water was measured and found to be 0.7 g/L. This compound would be classified as insoluble.
(True/False)
4.8/5  (36)
(36)
A solution has a concentration of 16 ppm. Which of the following is another way to describe the concentration of this solution?
(Multiple Choice)
4.7/5  (39)
(39)
Solution contains 55 mg of magnesium in 2.5 L of solution. The concentration of this solution is 2.2 mg/dL.
(True/False)
4.7/5  (44)
(44)
In cases of cerebral endema, a hypertonic solution is administered. The goal of giving the patient the hypertonic solution would be to pull fluid from the cells through the process of osmosis.
(True/False)
4.8/5  (38)
(38)
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.10 M in fructose and 0.05 M in glucose. Solution B is 0.20 M in sucrose. The direction of osmosis will be to the _______________________.
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.10 M in fructose and 0.05 M in glucose. Solution B is 0.20 M in sucrose. The direction of osmosis will be to the _______________________.
(Short Answer)
4.9/5  (41)
(41)
The osmotic pressure of a 0.10 M NaCl solution will be the same as that of a 0.10 M urea solution.
(True/False)
4.8/5  (34)
(34)
A solution is made by dissolving 22.5 mL of oil in enough gasoline to give 60.5 mL of solution. What is the % (v/v) of oil in the solution?
(Multiple Choice)
4.7/5  (39)
(39)
For the following questions,fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
-For many ionic solids such as NaHCO3 water solubility will ___________ at higher temperatures..
(Short Answer)
4.8/5  (33)
(33)
Showing 41 - 60 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)