Exam 5: Solution Concentration
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
The conversion factor for converting from moles of HPO42-- to equivalents is: 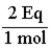
(True/False)
4.8/5  (27)
(27)
An oral rehydration solution contains 30 mEq/L of citrate3-, what is the molarity of citrate in this solution?
(Multiple Choice)
4.9/5  (42)
(42)
A solution contains 5.75 mg of magnesium ions in 332 mL of solution. What is the concentration of the solution in mEq/L?
(Multiple Choice)
4.7/5  (35)
(35)
Based on the following graph, the solubility of this substance at 80 °C is approximately: 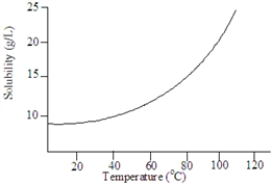
(Multiple Choice)
4.9/5  (37)
(37)
Consider the solutions shown in the containers below.The composition of each solution is given in the image. 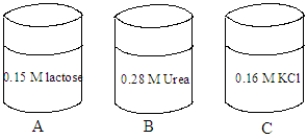 Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
-A red blood cell is placed in the solution in container B. The cell will undergo ____________________.
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
-A red blood cell is placed in the solution in container B. The cell will undergo ____________________.
(Short Answer)
4.9/5  (37)
(37)
What is the mass of salt in a 400.0 gram-sample of salt water which is 1.50% (w/w) salt?
(Multiple Choice)
4.9/5  (30)
(30)
For the following questions,fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
-When a beverage can is opened the pressure of the gas above the liquid will___________ causing the solubility of the gas to ____________.
(Short Answer)
4.9/5  (49)
(49)
A stockroom attendant has a 15.0% (w/v) solution of KOH. What volume of this solution should she use if she needs to prepare 20.0 mL of a 10.0% (w/v) solution?
(Multiple Choice)
4.8/5  (40)
(40)
If 1.00 mol of each of the following solutes is dissolved in 2.00 L of an aqueous solution, which solution contains the largest number of solute particles?
(Multiple Choice)
4.9/5  (38)
(38)
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
-Upon standing the water molecules will move in which direction?
Answer the following questions as appropriate with: right,left,or no movement.
-Upon standing the water molecules will move in which direction?
(Short Answer)
4.9/5  (29)
(29)
There is a 9 M aqueous HCl solution in the stock room, but a 5 M solution is required for an experiment. Doubling the volume of the 9 M sample with water will produce the 5 M solution.
(True/False)
4.9/5  (38)
(38)
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
-Calculate the percent concentration (m/v) of a solution prepared by dissolving 6.45 g of glucose in enough water to produce 85.0 mL of solution?
Answer the following questions as appropriate with: right,left,or no movement.
-Calculate the percent concentration (m/v) of a solution prepared by dissolving 6.45 g of glucose in enough water to produce 85.0 mL of solution?
(Short Answer)
4.7/5  (32)
(32)
The concentration of cholesterol in plasma was determined to be 215 mg/dL. The mass of cholesterol in 59.1 mL of this plasma is 127 mg.
(True/False)
4.7/5  (39)
(39)
Which of the following pass through both osmotic and dialysis membranes?
(Multiple Choice)
5.0/5  (30)
(30)
For a 2.00 M solution, the conversion factor for determining the number of moles of solute in a given volume of solution is: 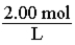
(True/False)
4.9/5  (39)
(39)
If the seminpermeable membrane has pores too small to allow glucose to pass through, ______________will not occur.
(Short Answer)
4.7/5  (43)
(43)
Based on the following graph, approximately what minimum temperature would be needed to dissolved 20 g of this solute in 1.00 L of water? 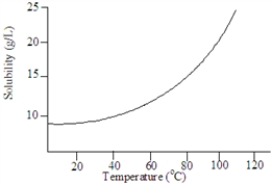
(Multiple Choice)
4.8/5  (38)
(38)
Consider the following structure. 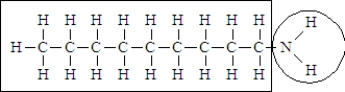 Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
-The portion of the molecule in the box is classified as ______________________ and that in the circle is classified as_____________________.
Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
-The portion of the molecule in the box is classified as ______________________ and that in the circle is classified as_____________________.
(Short Answer)
4.8/5  (33)
(33)
Showing 21 - 40 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)