Exam 5: Solution Concentration
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
The total molarity of an intravenous solution is given on the label as 151 mEq/L. The osmolarity of this solution is also 151 mEq/L.
(True/False)
4.9/5  (33)
(33)
What is the molarity of a solution containing 0.585 mol of lactic acid in 250.0 mL of solution?
(Multiple Choice)
4.7/5  (38)
(38)
A student is preparing a sugar water solution to make rock candy. When the student adds sugar to the solution, the added sugar does not dissolve. Which kind of solution does the student have?
(Multiple Choice)
4.7/5  (40)
(40)
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown. 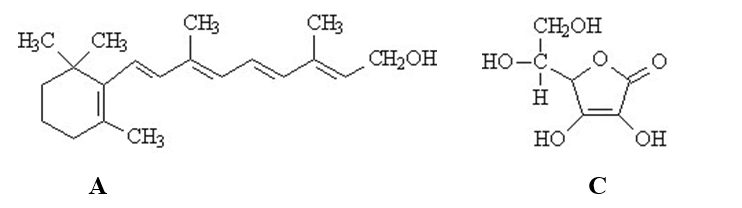 Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The vitamin that would be the most hydrophobic is ______.
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The vitamin that would be the most hydrophobic is ______.
(Short Answer)
4.9/5  (42)
(42)
If you have 9 g of NaCl in 1 L of water, this solution is 0.9% NaCl and is called normal saline or NS.
(True/False)
4.8/5  (34)
(34)
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 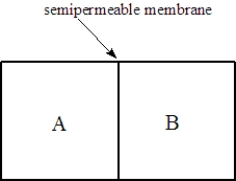 Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.10 M in lactose and 0.050 M in urea. Solution B is 0.15 M in KCl. Water will flow to the ______________________compartment.
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.10 M in lactose and 0.050 M in urea. Solution B is 0.15 M in KCl. Water will flow to the ______________________compartment.
(Short Answer)
4.9/5  (27)
(27)
If a solution contains 3.25 mol of Al3+, how many equivalents of Al3+ are present?
(Multiple Choice)
4.8/5  (27)
(27)
Putting a celery stick in distilled water results in the uptake of water by the celery.
(True/False)
4.9/5  (38)
(38)
Lactated Ringer's solution 109 mEq/L of Cl-. What is the mass of the chloride ion in 225 mL of this solution?
(Multiple Choice)
4.9/5  (33)
(33)
Consider the solutions shown in the containers below.The composition of each solution is given in the image. 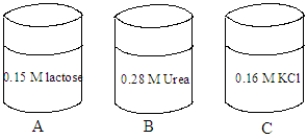 Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
-A red blood cell is placed in the solution in container A. The cell will undergo _____________________.
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
-A red blood cell is placed in the solution in container A. The cell will undergo _____________________.
(Short Answer)
4.8/5  (38)
(38)
A normal saline solution is a 0.90% (w/v) aqueous solution of NaCl. This is the same as: 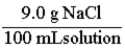
(True/False)
4.8/5  (42)
(42)
What will happen to a red blood cell if placed in the following solution? 0.14 M lactose (a nonelectrolyte)
(Multiple Choice)
4.8/5  (38)
(38)
Consider the two containers shown below separated by a seminpermeable membrane. When allowed to stand overnight,  there will be no change in the liquid levels.
there will be no change in the liquid levels.
(True/False)
4.8/5  (40)
(40)
Which of the following is associated with cells in a hypertonic solution?
(Multiple Choice)
4.9/5  (36)
(36)
Consider the two containers shown below separated by a seminpermeable membrane. When allowed to stand overnight,  water will have moved from the right-hand container to the left hand container..
water will have moved from the right-hand container to the left hand container..
(True/False)
4.9/5  (36)
(36)
Showing 61 - 76 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)