Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Which of the following could NOT be represented by a conceptual model?
(Multiple Choice)
4.8/5  (39)
(39)
If a neutral element has the following chemical notation, how many electrons does it have? fluorine-19
(Multiple Choice)
5.0/5  (32)
(32)
An atom absorbs or emits only particular frequencies of light. White light bends into a glass prism and separates into a rainbow of colors. In which of these two scenarios does the electromagnetic radiation behave as a wave? As a particle?
(Multiple Choice)
4.9/5  (44)
(44)
A one ounce Ping-Pong ball is pushed upwards by a fan so that the Ping-Pong ball hovers neither rising nor falling. What is the force of the wind on the Ping-Pong ball in units of ounces?
(Multiple Choice)
5.0/5  (34)
(34)
From which of the following atoms would removing an electron be the easiest?
(Multiple Choice)
4.8/5  (40)
(40)
If an element has 9 protons and 10 neutrons and 9 electrons, which expression correctly identifies the element?
(Multiple Choice)
4.8/5  (32)
(32)
An element has two different isotopes: one that weighs 65 amu and another that weighs 67 amu. If the average atomic mass of all the isotopes is 66.5 amu, what can be said about the relative abundance of the isotopes?
(Multiple Choice)
4.9/5  (43)
(43)
What does the following element description actually mean? 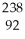 U
U
(Multiple Choice)
4.8/5  (37)
(37)
In the following diagram, which of the following measurements would represent the distance the wave travels in 1 second if it has a frequency of 0.5 Hz? 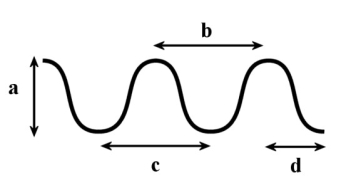
(Multiple Choice)
4.7/5  (40)
(40)
If a neutral element has the following chemical notation, how many electrons does it have? carbon-13
(Multiple Choice)
4.9/5  (38)
(38)
The following statement describes which subatomic particle best? It is located outside of the nucleus.
(Multiple Choice)
4.7/5  (41)
(41)
If an element has 15 protons and 16 neutrons and 15 electrons, what is the atomic mass of the element?
(Multiple Choice)
4.9/5  (38)
(38)
The nucleus of an electrically neutral iron atom contains 26 protons. How many electrons does this iron atom have?
(Multiple Choice)
4.8/5  (45)
(45)
How does Rutherford's model of the atom explain why some of the alpha particles directed at the gold foil were deflected straight back toward the source?
(Multiple Choice)
4.8/5  (35)
(35)
Why did Rutherford propose a model for the atom with a very small nucleus containing the bulk of the mass and a positive charge?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 41 - 60 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)