Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
A beam of protons and a beam of neutrons of the same energy are both harmful to living tissue. The beam of neutrons, however, is less harmful. Why?
(Multiple Choice)
4.9/5  (37)
(37)
An electron in an outermost shell has more energy than one in an innermost shell because ________.
(Multiple Choice)
4.8/5  (40)
(40)
Does it make sense to say that a textbook is about 99.9 percent empty space?
(Multiple Choice)
4.8/5  (42)
(42)
If two protons and two neutrons are removed from the nucleus of the oxygen-16 isotope, a nucleus of which element remains?
(Multiple Choice)
4.9/5  (38)
(38)
Since atoms are mostly empty space, why don't objects pass through one another?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following statements about electrons is true?
(Multiple Choice)
4.8/5  (41)
(41)
What does the following element description actually mean? hydrogen-2
(Multiple Choice)
4.7/5  (34)
(34)
Another interesting periodic trend is the density of the elements. We find that osmium, Os (atomic number 76)has the greatest density of all elements, and, with some exceptions, the closer an element is positioned to osmium, the greater its density. Based upon this trend, list the following elements in order of increasing density: copper, Cu, gold, Au, platinum, Pt, and silver, Ag.
(Multiple Choice)
4.8/5  (29)
(29)
Which of these shows light waves in order of increasing energy?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following is not a form of electromagnetic radiation?
(Multiple Choice)
4.9/5  (29)
(29)
If a neutral element has the following chemical symbol, how many electrons does it have? 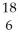 O
O
(Multiple Choice)
4.9/5  (33)
(33)
What property of an electron makes it possible to use electron microscopes?
(Multiple Choice)
4.7/5  (48)
(48)
If a neutral element has the following chemical symbol, how many electrons does it have? 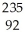 U
U
(Multiple Choice)
4.8/5  (46)
(46)
Which of the following determined the ratio of the charge of an electron to its mass?
(Multiple Choice)
4.8/5  (38)
(38)
In some instances electromagnetic radiation behaves like a wave. In other instances electromagnetic radiation behaves more like a particle. Which behavior more accurately describes the true nature of electromagnetic radiation?
(Multiple Choice)
4.8/5  (33)
(33)
Which color of light comes from the higher energy transition, red or blue?
(Multiple Choice)
4.9/5  (27)
(27)
Showing 121 - 140 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)