Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Would you use a physical model or a conceptual model to describe the following: a gold coin, dollar bill, car engine, air pollution, virus, spread of sexually transmitted disease?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following statements best describes electromagnetic radiation?
(Multiple Choice)
4.8/5  (32)
(32)
The element bromine, Br (atomic number 35), has two major isotopes of similar abundance, both around 50 percent. The atomic mass of bromine is reported in the periodic table as 79.904 atomic mass units. Choose the most likely set of mass numbers for these two bromine isotopes.
(Multiple Choice)
4.9/5  (34)
(34)
Why are the atomic masses listed in the periodic table not whole numbers?
(Multiple Choice)
4.8/5  (38)
(38)
About how many times larger is a bacterium compared to an atom?
(Multiple Choice)
4.8/5  (37)
(37)
How is it possible for an atom to emit visible light even though the atom is smaller than the wavelength of visible light?
(Multiple Choice)
4.8/5  (41)
(41)
Suppose that a certain atom possesses only four distinct energy levels. Assuming that all transitions between levels are possible, how many spectral lines will this atom exhibit? 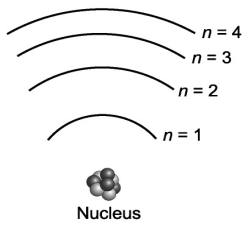
(Multiple Choice)
5.0/5  (38)
(38)
Which of the following might best explain the reason why electrons are restricted to certain energy levels in an atom?
(Multiple Choice)
4.9/5  (46)
(46)
If an element has 10 protons and 11 neutrons and 10 electrons, which expression correctly identifies the element?
(Multiple Choice)
4.8/5  (29)
(29)
If the particles of a cathode ray had a greater electric charge, the ray passing through a magnetic field would bend ________.
(Multiple Choice)
4.7/5  (40)
(40)
In the following diagram, which of the following is the measurement of one wavelength? 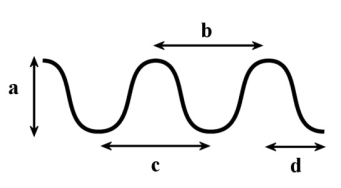
(Multiple Choice)
4.8/5  (41)
(41)
What is the approximate mass of a carbon atom in atomic mass units (amu)? How about a carbon dioxide molecule?
(Multiple Choice)
4.8/5  (33)
(33)
What is the relationship between the light emitted by an atom and the energies of the electrons in the atom?
(Multiple Choice)
4.8/5  (35)
(35)
When we breathe we inhale oxygen, 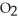 , and exhale carbon dioxide,
, and exhale carbon dioxide, 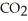 , plus water vapor,
, plus water vapor, 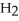 O. Which likely has more mass, the air that we inhale or the same volume of air we exhale? Does breathing cause you to lose or gain weight?
O. Which likely has more mass, the air that we inhale or the same volume of air we exhale? Does breathing cause you to lose or gain weight?
(Multiple Choice)
4.9/5  (24)
(24)
Using the following generic atom description, choose the correct method for determining the number of neutrons. 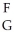 X
X
(Multiple Choice)
4.8/5  (38)
(38)
The ray of light within a neon sign bends when a magnet is held up to it because ________.
(Multiple Choice)
4.8/5  (42)
(42)
What was Niels Bohr's explanation for the observation of atomic spectra?
(Multiple Choice)
4.9/5  (34)
(34)
How is it possible to deduce the identity of an element from its electron configuration?
(Multiple Choice)
5.0/5  (40)
(40)
Showing 81 - 100 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)