Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Boron has primarily two isotopes, one with an atomic mass of 11.0 amu and another with an atomic mass of 10.0 amu. If the abundance of the boron atom with a mass of 11.0 amu is 18.9 percent and the abundance of the other isotope is 81.1 percent, what would be the average mass of the boron atom?
(Multiple Choice)
4.7/5  (38)
(38)
An element found in another galaxy exists as two isotopes. If 80.0 percent of the atoms have an atomic mass of 80.00 amu and the other 20.0 percent have an atomic mass of 82.00 amu, what is the atomic mass of the element?
(Multiple Choice)
5.0/5  (27)
(27)
What color do you see when you close your eyes while in a dark room? Explain.
(Multiple Choice)
4.8/5  (38)
(38)
What does the following element description actually mean? iron-57
(Multiple Choice)
4.8/5  (37)
(37)
Which has more atoms: a 1-gram sample of carbon-12 or a 1-gram sample of carbon-13?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following gases would diffuse the fastest through an extremely fine membrane?
(Multiple Choice)
4.9/5  (38)
(38)
It is relatively easy to pull an electron away from a potassium atom (K, atomic number 19), but very difficult to remove a second one because ________.
(Multiple Choice)
4.7/5  (33)
(33)
What do the electron configurations for all the group 18 noble gases have in common?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following diagrams best represents the size of the atomic nucleus relative to the size of the atom? 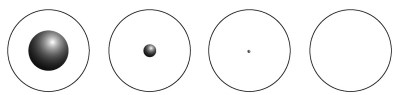 A B C D
A B C D
(Multiple Choice)
4.9/5  (35)
(35)
What prompted early scientists to propose that the ray of the cathode ray tube was actually a negatively charged particle?
(Multiple Choice)
4.8/5  (33)
(33)
The following statement describes which subatomic particle best? It is a nucleon.
(Multiple Choice)
4.8/5  (38)
(38)
The isotope lithium-7 has a mass of 7.0160 atomic mass units, and the isotope lithium-6 has a mass of 6.0151 atomic mass units. Given the information that 92.58 percent of all lithium atoms found in nature are lithium-7 and 7.42 percent are lithium-6, calculate the atomic mass of lithium, Li (atomic number 3).
(Multiple Choice)
4.8/5  (40)
(40)
Neon, Ne, (number 10)has a relatively large effective nuclear charge, yet it cannot attract any additional electrons because ________.
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following best describes a conceptual model of an atom?
(Multiple Choice)
4.8/5  (40)
(40)
The following statement describes which subatomic particle best? It has a relatively large mass.
(Multiple Choice)
4.9/5  (34)
(34)
Showing 61 - 80 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)