Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Which of the following statements is not true about a neutralization reaction?
(Multiple Choice)
4.7/5  (37)
(37)
At what point will a buffer solution cease to moderate changes in pH?
(Multiple Choice)
4.8/5  (36)
(36)
When the hydronium ion concentration equals 10 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
5.0/5  (46)
(46)
Does a water molecule become more or less polar when bound to a metal ion, such as 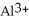 ? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
(Multiple Choice)
4.8/5  (30)
(30)
If the pH of a solution is 10, what is the hydronium ion concentration?
(Multiple Choice)
4.8/5  (43)
(43)
For the following acid-base reaction, identify what compound is formed in the space marked. HCl + KOH ⇌ ???? + H2O
(Multiple Choice)
4.9/5  (37)
(37)
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
(Multiple Choice)
4.9/5  (40)
(40)
An acid and a base react to form a salt, which consists of positive and negative ions. Which forms the positive ions: the acid or the base? Which forms the negative ions?
(Multiple Choice)
4.9/5  (25)
(25)
How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Why? 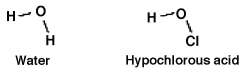
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following compounds could never act as an acid?
(Multiple Choice)
4.9/5  (31)
(31)
What is the hydroxide ion concentration in an aqueous solution when the hydronium ion concentration equals 1 × 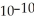 moles per liter?
moles per liter?
(Multiple Choice)
4.8/5  (35)
(35)
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-5M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.7/5  (36)
(36)
What is the hydroxide ion concentration in an aqueous solution where the pH = 5?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following items would you use to make an acidic buffer solution?
(Multiple Choice)
4.8/5  (39)
(39)
If you had a 1 M solution of a strong acid what would be its pH?
(Multiple Choice)
4.9/5  (51)
(51)
Showing 61 - 80 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)