Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Pour vinegar onto beach sand from the Caribbean and the result is a lot of froth and bubbles. Pour vinegar onto beach sand from California, however, and nothing happens. Why?
(Multiple Choice)
5.0/5  (38)
(38)
Which should be a stronger base: ammonia, 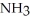 , or trifluoronitrogen,
, or trifluoronitrogen, 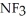 ? Why?
? Why? 
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following statements describes a basic solution?
(Multiple Choice)
4.9/5  (30)
(30)
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
(Multiple Choice)
4.8/5  (37)
(37)
Sodium hydroxide is added to a buffer solution of ammonia, N 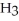 , and ammonium chloride,
, and ammonium chloride, 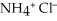 . What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
. What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
(Multiple Choice)
4.7/5  (44)
(44)
What happens to the pH of soda water as it loses its carbonation?
(Multiple Choice)
5.0/5  (38)
(38)
The overall rate at which humans are producing carbon dioxide is ________.
(Multiple Choice)
4.9/5  (25)
(25)
In the following buffer system, what happens to the concentration of the highlighted molecule if you add acid in the form of H3O+? HF + NaF + H2O ⇌ F- + H3O+ + Na+
(Multiple Choice)
4.8/5  (41)
(41)
Which of the above illustrations shows a neutral aqueous solution?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following items would you use to make a basic buffer solution?
(Multiple Choice)
4.9/5  (40)
(40)
If the pH of a solution is 10, what is the hydroxide ion concentration?
(Multiple Choice)
4.9/5  (34)
(34)
When a hydronium ion concentration equals 1 × 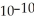 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.8/5  (35)
(35)
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-1M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.9/5  (38)
(38)
In the reaction below, what does the symbol ⇌ mean? OH- + NH4+ ⇌ H2O + NH3
(Multiple Choice)
4.9/5  (41)
(41)
Arrange the following images of an aqueous base solution in order of increasing base strength: 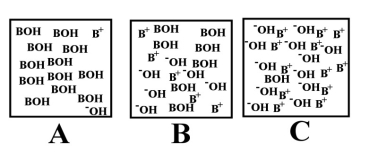
(Multiple Choice)
4.8/5  (22)
(22)
How might you tell whether or not your toothpaste contained either calcium carbonate, CaC 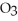 , or baking soda, NaHC
, or baking soda, NaHC 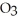 , without looking at the ingredients label?
, without looking at the ingredients label?
(Multiple Choice)
4.7/5  (33)
(33)
When the pH of a solution is 1, the concentration of hydronium ions is 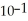 M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
(Multiple Choice)
4.7/5  (34)
(34)
Showing 41 - 60 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)