Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Sodium hydroxide, NaOH, is a strong base, which means that it readily accepts hydrogen ions. What products are formed when sodium hydroxide accepts a hydrogen ion from a water molecule?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following statements about strong or weak acids is true?
(Multiple Choice)
4.8/5  (37)
(37)
If we were to lower our rate of CO2 production by a third-down from 9 to 6 billion tons per year-the concentration of CO2 in the atmosphere should ________.
(Multiple Choice)
4.8/5  (34)
(34)
Water is formed from the reaction of an acid and a base. Why is it not classified as a salt?
(Multiple Choice)
4.8/5  (36)
(36)
What atom in the ammonium ion, 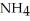 ⁺ bears the positive charge?
⁺ bears the positive charge?
(Multiple Choice)
4.8/5  (40)
(40)
What do the brackets in the following equation represent? [H3O+] × [OH-] = Kw
(Multiple Choice)
4.8/5  (39)
(39)
According to the following reaction, which molecule is acting as a base? OH- + NH4+ → H2O + NH3
(Multiple Choice)
4.9/5  (42)
(42)
What does the value of 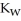 say about the extent to which water molecule react with themselves?
say about the extent to which water molecule react with themselves?
(Multiple Choice)
4.7/5  (41)
(41)
According to the following reaction, which molecule is acting as an acid? H2O + NH3 → OH- + NH4+
(Multiple Choice)
4.7/5  (35)
(35)
Sodium hydroxide, NaOH, is a very strong base. If a concentrated solution of this base were to spill on a latex glove you were wearing, it would feel like regular water. If the solution were to land directly on your skin, however, it would feel very slippery. Why?
(Multiple Choice)
4.8/5  (33)
(33)
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
(Multiple Choice)
4.7/5  (42)
(42)
According to the following reaction, which molecule is acting as an acid? OH- + NH4+ → H2O + NH3
(Multiple Choice)
4.8/5  (43)
(43)
For the following acid-base reaction, identify what compound is formed in the space marked. HNO3 + KOH ⇌ ???? + H2O
(Multiple Choice)
4.7/5  (46)
(46)
Some molecules are able to stabilize a negative charge by passing it from one atom to the next by a flip-flopping of double bonds. This occurs when the negative charge is one atom away from an oxygen double bond as follows. Note that the curved arrows indicate the movement of electrons: 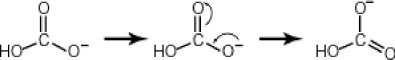 Why then is sulfuric acid so much stronger an acid than carbonic acid?
Why then is sulfuric acid so much stronger an acid than carbonic acid? 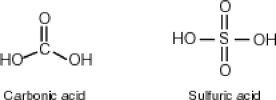
(Multiple Choice)
4.7/5  (35)
(35)
Why might an area with a large amount of limestone (CaCO3)be less susceptible to acid rain?
(Multiple Choice)
4.8/5  (31)
(31)
According to the following reaction, which molecule is acting as an acid? H2O + H2SO4 → H3O+ + HSO4-
(Multiple Choice)
4.8/5  (41)
(41)
Showing 21 - 40 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)